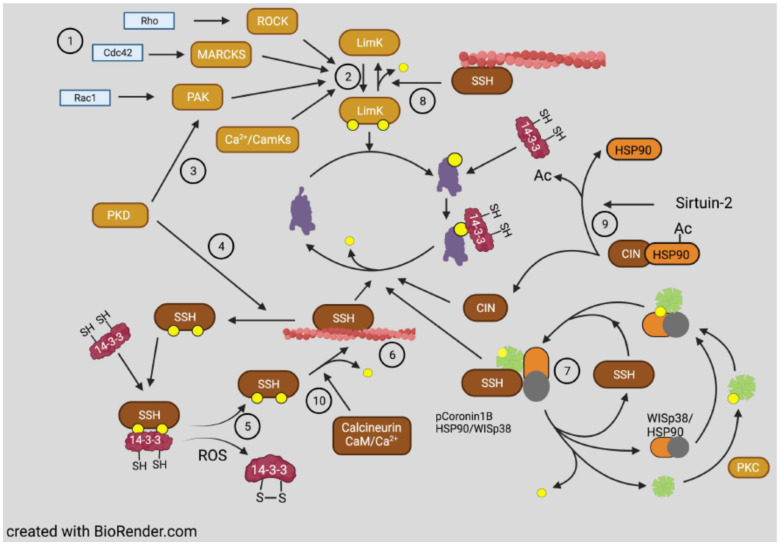
Figure 5.
Major pathways for phospho-regulation of cofilin. ① Upstream activation of PAKs by Cdc42/Rac relieves autoinhibition allowing self-phosphorylation of ser474 (PAK4) with subsequent phosphorylation (activation) of LIMK1/2 (T508 and T505, respectively) ②. Protein kinase D1 (PKD) ③ phosphorylates PAK4 on ser99, which mediates its binding to 14-3-3 (not shown) and recruits it to sites of LIMK1 at the membrane [90,91]. Two sites in the C-terminal tail domain of SSH1L are phosphorylated. PKD phosphorylates SSH1L on S978 ④, which enhances binding of 14-3-3 to inhibit dephosphorylation by non-specific phosphatases and inhibits its F-actin binding through its tail domain, one mechanism by which its N-terminal cofilin phosphatase activity is activated [92,93]. PKD isoforms, thus, serve as rapid inactivators (phosphorylation) of localized cofilin activity [91,94]. Removal of 14-3-3 from phospho-SSH1 ⑤ can be achieved by oxidation (peroxide or ROS from NOX), causing disulfide bond formation within 14-3-3 [95]. SSH1 requires binding to F-actin ⑥ or ⑦ to a complex of phospho-coronin1B (green molecule) bound to WISp38/Hsp90 [89]. Active SSH1 also serves to dephosphorylate LIMK1 on T508 ⑧, which can bring about a rapid increase in active cofilin [88]. Much of CIN in neurons is held in an inactive form by hsp90 but can be released in an active state ⑨ by hsp90 inhibitor 17-AAG [96] or possibly by sirtuin-2 (an HDAC) deacetylation of hsp90 [97]. Calcineurin, a Ca2+/calmodulin-activated phosphatase, also activates ⑩ SSH1 [98], the likely mechanism of SSH1 activation through integrin/RanBP9 [99].