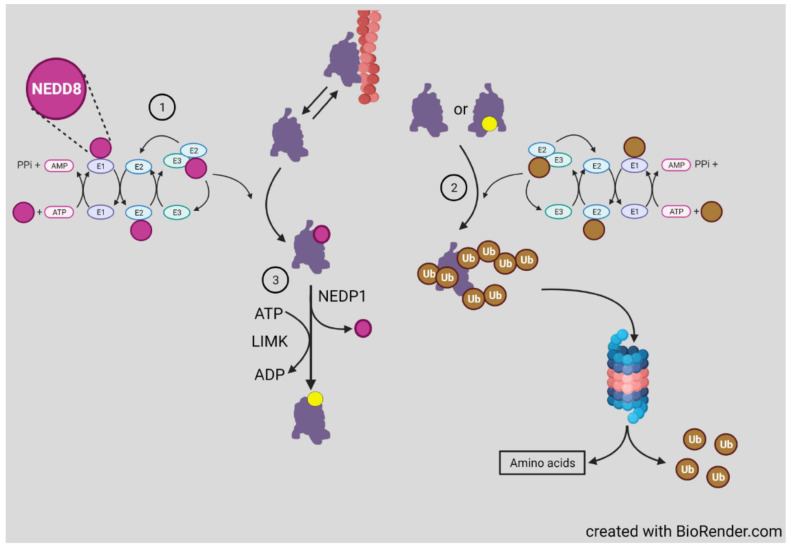
Figure 6.
Enzymatic cascades for ubiquitinylation and neddylation of cofilin. ① Specific enzymes (E1), for which there is often only a single isoform, activate the polypeptide modifier, ubiquitin, or NEDD8 through a thioacyl derivative on its C-terminus linking it to E1. The activated polypeptide is then transferred to an E2 enzyme for which a few different isoforms are expressed. The E3 enzyme, which exists as multiple isoforms with different substrate recognition capabilities, binds both its substrate and the E2-polypeptide and transfers the polypeptide to its lysine acceptor in the substrate. Neddylation targets cofilin on only K112, preventing it from rapidly rebinding F-actin and allowing time for its phosphorylation [108]. ② Cofilin K112 and lysines 19, 92, 144, and 164 are ubiquitinylated, a process enhanced by phosphorylation of Y68 by vSrc tyrosine kinase, resulting in more rapid cofilin degradation by the proteasome [107]. The NEDD8 activating enzyme is inhibited by MLN4924, and NEDD8 is removed from substrates ③ by the enzyme NEDP1. Inhibition of neddylation results in a large increase in F-actin bound cofilin due to its decreased phosphorylation.