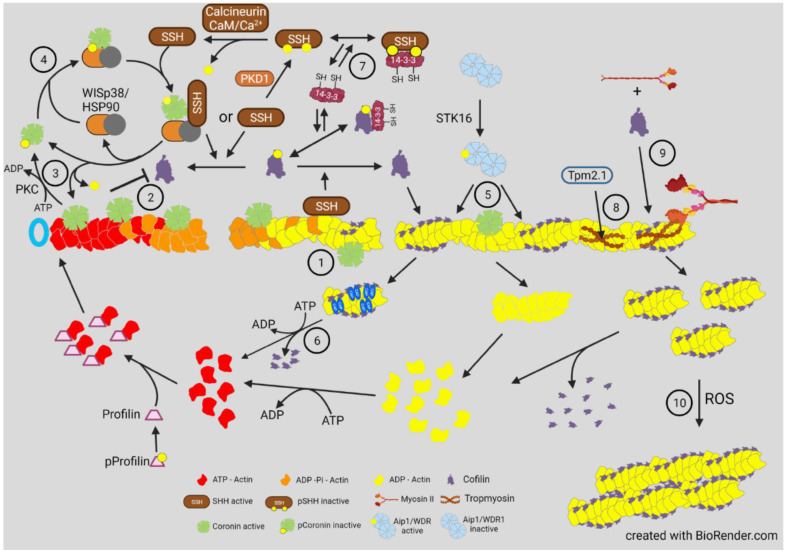
Figure 7.
Proteins modulating the turnover of F-actin with cofilin. Coronin’s have multiple roles in actin turnover in terms of being able ① to recruit cofilin to ADP-actin subunits and ② to inhibit cofilin binding to ATP/ADP-Pi actin subunits while enhancing Arp2/3 complex binding (not shown) [159]. ③ When phosphorylated by protein kinase C (PKC) on ser2, coronin1B dissociates from filaments and either remains inactive when bound to 14-3-3 until dephosphorylated by SSH [162], or ④ binds a protein scaffold of WISp39/Hsp90, which can then recruit and activate SSH to dephosphorylate cofilin as well as itself [87,89], disrupting the complex and releasing active coronin1B. Dephosphorylated coronin1B can also bind the “aged” ADP-subunits in F-actin, where it serves to recruit cofilin and enhances turnover [160,161]. Aip1, when phosphorylated by the constitutively active STK16, enhances severing ⑤, and CAP1/2 dimer/oligomers (dark blue) enhance cofilin release and nucleotide exchange on released actin monomers ⑥. 14-3-3 proteins ⑦ are modulators of phosphoprotein mediated actin dynamics, inhibiting dephosphorylation of several proteins by non-specific phosphatases [81,93,162]. Thus, 14-3-3 proteins are major integrators of phosphoprotein cycles that drive actin dynamics. Different Tpm isoforms ⑧ can inhibit or permit cofilin binding [163]. The Tpm 2.1 isoform allows either cofilin or myoII binding ⑨, which compete in modulating myoII-dependent contractile processes in cells. ⑩ Fragments of cofilin-saturated F-actin can associate under oxidative conditions to form cofilin-actin rods.