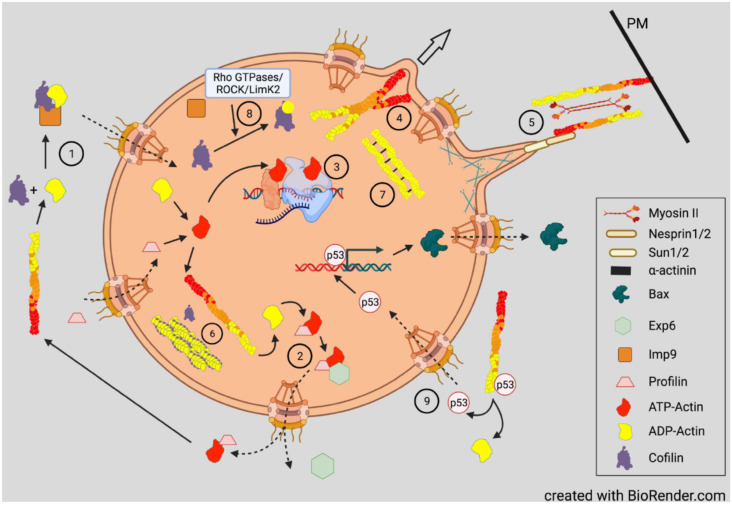
Figure 8.
Nuclear uptake and nuclear functions of cofilin. Cofilin can chaperone actin transport into the nucleus ① via Importin 9, probably as ADP-actin to which it has much higher affinity than to ATP-actin. Export of actin ② is via profilin-actin-Exportin 6. ③ Nuclear actin is a required subunit in RNA polymerases and chromatin remodeling factors [240]. Forces within the nucleus that result in nuclear envelope protrusions ④ are driven by actin assembly [241], but strong cytoplasmic forces ⑤ by filaments linking the nuclear matrix through the nuclear envelope via SUN1/2 and nesprin1/2 also result in nuclear deformations that are controlled by cofilin competing with cytoplasmic myoII [176]. Actin rods in the nucleus ⑥ form under stress and may contain cofilin (heat shock stress) as well as being formed from αlpha-actinin and actin ⑦, especially prevalent in some muscle diseases [242]. α-Actinin-4 mediates gene expression for proliferation by binding beta-catenin, which is an activator of the wnt signaling pathway for cell proliferation. Rods that form under stress tie up the α-actinin, blocking this cell proliferation pathway. Thus, rod formation might be a rapid and efficient method for sequestering proteins in response to stress. ⑧ Nuclear Rho GTPases can signal via ROCK/PAKs to LIMK2 to inhibit proliferation (a decline in LIMK 2 enhances tumor progression via beta-catenin and wnt signaling). Active LIMK2 causes cell cycle arrest at the G1/S transition. Nuclear uptake ⑨ of p53, a tumor suppressing protein that stimulates the production of BAX and other proapoptotic genes, is regulated in part by cytoplasmic F-actin [243,244] and cofilin-mediated depolymerization aids in p53 nuclear translocation. Further details on nuclear actin may be found in [240,241].