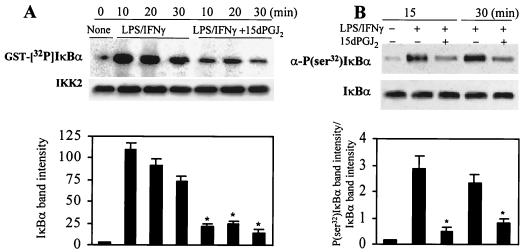
FIG. 6.
Inhibition of IκBα phosphorylation in activated RAW 264.7 cells treated with 15dPGJ2. Macrophages were incubated with 2 μM 15dPGJ2 5 min prior to stimulation with LPS (200 ng/ml) and IFN-γ (10 U/ml). At the indicated times, cell extracts were prepared, IKK was immunoprecipitated, and the in vitro kinase activity of 100 ng of IP protein was assayed using GST-IκBα(1-317) and [γ-32P]ATP as substrates. (A) After 10 min of incubation, GST-IκBα was purified with glutathione-agarose and analyzed by SDS-PAGE (10% gel). Aliquots (5 μl) of the kinase reaction mixture were analyzed by Western blotting to determine the amount of IKK2 present in each assay. (B) Cells treated with 10 μM MG132 were stimulated as described previously; at the indicated times, cytosolic extracts were prepared and the amount of endogenous P(Ser32)IκBα was determined using a specific Ab. The blot was reprobed with anti-IκBα Ab. The intensity of the bands of phosphorylated IκBα (A) and the ratio between the band intensities of P(Ser32)IκBα and IκBα (B) are given. Results show the mean ± SEM of three experiments. ∗, P < 0.005 with respect to the corresponding LPS/IFN-γ condition.