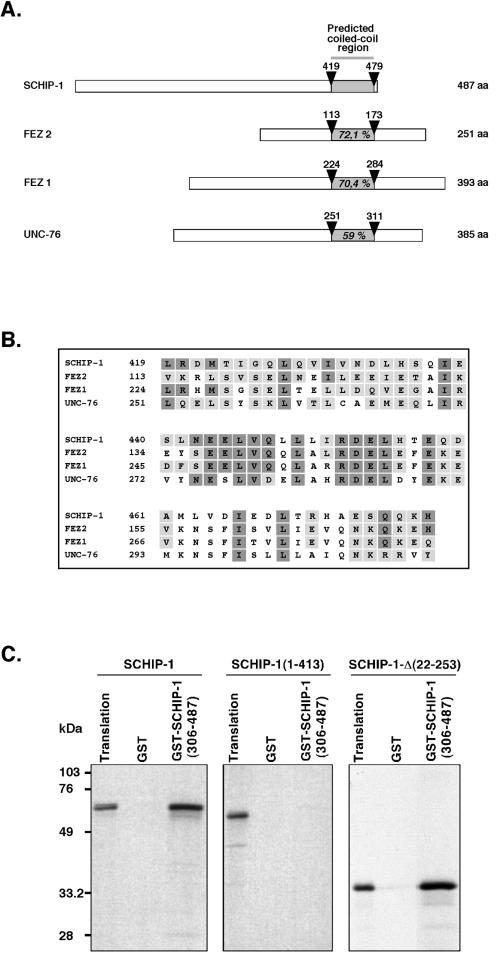
FIG. 2.
SCHIP-1 can homodimerize through its coiled-coil domain homologous to the FEZ and UNC-76 proteins. (A) Schematic alignment of human SCHIP-1 with human FEZ1 and FEZ2 and C. elegans UNC-76 proteins. Open boxes represent full-length SCHIP-1, FEZ1, and UNC-76 proteins and the 251 C-terminal amino acids (aa) of the FEZ2 protein. The grey color highlights the localization of the coiled-coil domain in each protein. Arrowheads indicate amino acids delimiting coiled-coil domains. Percentages of homology with SCHIP-1 in the coiled-coil domains are indicated. (B) Amino acid alignment of human SCHIP-1 with human FEZ1 and FEZ2 and C. elegans UNC-76 proteins within the predicted coiled-coil domain. Identical amino acids are presented in dark boxes; conserved residues are displayed in grey boxes. (C) The predicted coiled-coil domain is required for SCHIP-1 homodimerization. SCHIP-1 and SCHIP-1 deletion mutants were expressed in vitro in the presence of [35S]methionine and incubated in TKT150 buffer with either GST or GST–SCHIP-1(306-487) bound to glutathione-agarose beads. After washing, retained SCHIP-1 and SCHIP-1 variant proteins were eluted, resolved by SDS-PAGE, and visualized by autoradiography. Aliquots of the labeled proteins corresponding to 1/40 of the input were loaded on the same gel (Translation). The C-terminal region of SCHIP-1 including the coiled-coil domain [GST–SCHIP-1(306-487)] interacts with full-length SCHIP-1 or SCHIP-1-Δ(22-253) but not with a truncated SCHIP-1 missing the predicted coiled-coil domain [SCHIP-1(1-413)].