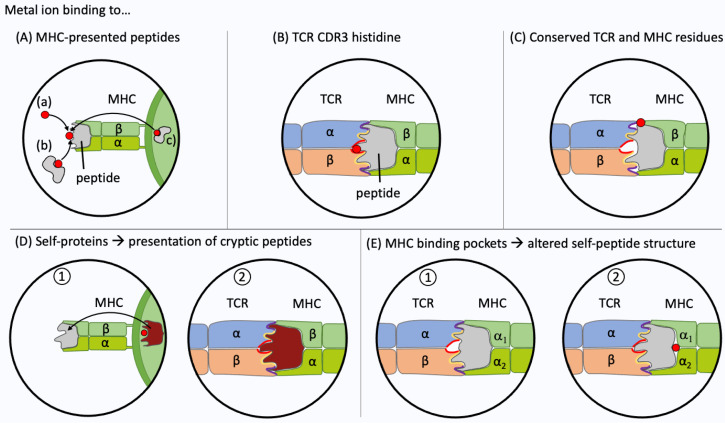
Figure 4.
Metal recognition by T cells. Experimental evidence has been obtained for five different interactions between the T cell receptor (TCR), the metal ion (highlighted in red) and the peptide presented at the surface of the major histocompatibility complex (MHC). (A) The metal ion haptenizes the presented peptide. Loading can occur directly from the extracellular space (a) or by transfer from a metal-binding protein [232] (b). The metal ion may also be pre-loaded to the MHC-presented peptide, e.g., by antigen-processing of a metal-binding protein (c). (B) Metal ion binding at the TCR complementarity-determining region 3 (CDR3). The metal ion may bind to the TCR CDR3. Recently, frequent Ni2+ binding via a histidine in the CDR3 of TCR α- or β-chain has been shown [115]. (C) Metal ion binding to conserved residues at the TCR-peptide-MHC interface. Ni2+ ions often bind via tyrosine36 in the CDR1 of TRAV9-2+ TCR and histidine81 in the MHC II β-chain [114,115]. (D) Recognition of cryptic epitopes. Metal ions may alter antigen-processing of a self-protein which in turn leads to the presentation of a metal-free cryptic self-peptide. (E) Metal ions may bind within the cleft between MHC and antigen peptide. The TCR is not activated by a self-pMHC complex (1). The metal ion binding leads to structural conformation changes of the presented peptide and creates a metal-free neo antigen recognized by the TCR (2). This type of interaction has been shown for beryllium (II) ions in combination with HLA-DP2 [233].