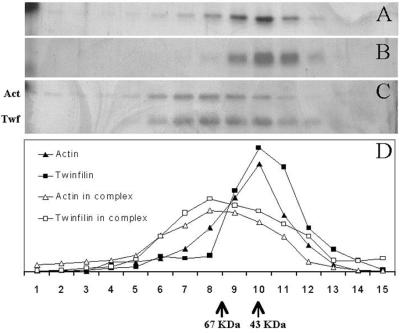
FIG. 4.
Rate zonal gradient centrifugation analysis of the stoichiometries of the interaction of human platelet nonmuscle actin and mouse A6/twinfilin. Samples in panels A (actin alone), B (A6/twinfilin alone), and C (actin and A6/twinfilin together) were floated independently but simultaneously in identical reaction conditions. In panel C, actin and A6/twinfilin were incubated together only during the 40-h centrifugation. (D) Graphic representation of distribution of proteins in panels A to C. Fractions from the left correspond to the heaviest or least globular proteins or protein complexes. Actin (42 kDa) and A6/twinfilin (40 kDa) alone peak at the same fraction as the 43-kDa size standard. Actin is distributed more broadly and toward larger molecular masses. This is understandable in the light the autoaffinity of actin and its ability to form filaments. A6/twinfilin does not seem to possess intrinsic affinity to itself under these conditions. In panel C, the distribution of actin and A6/twinfilin is broadened and shifted to the left by a distance that corresponds to a formation of a >67-kDa complex. The peak positions of the sedimentation assay protein standards (ovalbumin [43 kDa] and BSA [67 kDa]) are indicated at the bottom.