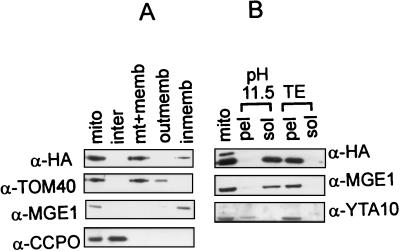
FIG. 5.
Biochemical fractionation localizes the Hmi1 protein in mitochondria. (A) Analysis of intracellular localization of the Hmi1 protein. Yeast cells (strain W303-1A), transformed with the expression construct YCAH-HMI1, and mitochondria were isolated by using fractionation by differential centrifugation. Mitochondria were subfractionated further, and the fractions were analyzed by Western blotting. The blots were probed with monoclonal anti-HA antibody 12CA5 (HA) or rabbit polyclonal antibodies against TOM40 (outer membrane protein), CCPO (intermembrane space protein), or MGE1 (peripheral inner membrane protein). Lane 1, mitochondria; lane 2, intermembrane space; lane 3, matrix plus membranes; lane 4, outer membrane; lane 5, inner membrane. (B) The Hmi1 protein is not an integral inner membrane component. Mitochondria were diluted, sonicated briefly, and extracted on ice with 0.1 M sodium carbonate (pH 11.5) (lanes 2 and 3) or with 10 mM Tris (pH 8.0)–1 mM EDTA (lanes 4 and 5). Soluble and membrane-bound fractions were separated by centrifugation for 1.5 h at 35,000 rpm in the Beckman Ti70 rotor. Pellet (pel) and supernatant (sol) fractions were analyzed by Western blotting with anti-HA monoclonal antibody 12CA5 and polyclonal antibodies against YTA10p (integral inner membrane protein) or Mge1p (peripheral inner membrane protein).