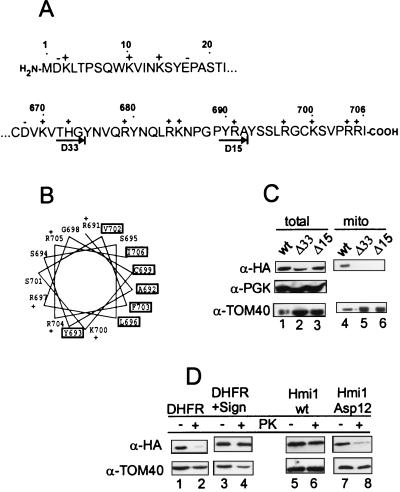
FIG. 7.
The C-terminal segment of the Hmi1 helicase is required for correct targeting of the protein into mitochondria. (A) Sequences of the N- and C-terminal segments of the Hmi1 protein. The acidic (−) and basic (+) amino acid residues are indicated above the sequence. The endpoints of the two C-terminal deletion mutants Mrh-ΔC15Ala and Mrh-ΔC33Gly are indicated with arrows below the C-terminal sequence segment. (B) Helical wheel presentation of the 18 C-terminal amino acid residues starting from Arg-691. Hydrophobic residues are indicated with boxes, and the positively charged residues are indicated with a “+.” (C) Western blot analysis of whole-cell extracts and mitochondrial fractions from the W303-1A strain transformants expressing the Hmi1 protein or the corresponding deletion mutant. Lanes 1 and 4, W303-1A transformed with YCAH-HMI1; lanes 2 and 5, W303-1A transformed with YCAH-ΔC33Gly; lanes 3 and 6, W303-1A transformed with YCAH-ΔC15Ala. The blots were probed with the anti-HA antibody 12CA5, anti-TOM40 (mitochondrial marker), and anti-PGK (cytoplasmic marker). (D) The C-terminal segment of the Hmi1 protein targets the DHFR carrier protein into mitochondria, and introduction of negatively charged residues in the C-terminus of the Hmi1 protein abolishes the mitochondrial import. The following proteins were expressed in W303-1A strain cells: lanes 1 and 2, HA-tagged DHFR; lanes 3 and 4, the fusion protein of HA-DHFR and the C-terminal segment (residues 616 to 706); lanes 5 and 6, HA-tagged Hmi1 protein; and lanes 7 and 8, mutant HA-Hmi1-Asp12. A Western blot shows analysis of total cellular protein (lanes 1, 3, 5, and 7) and the mitochondrial fraction treated with proteinase K for 10 min on ice (lanes 2, 4, 6, and 8). The blots were probed with anti-HA antibody 12CA5 and anti-TOM40.