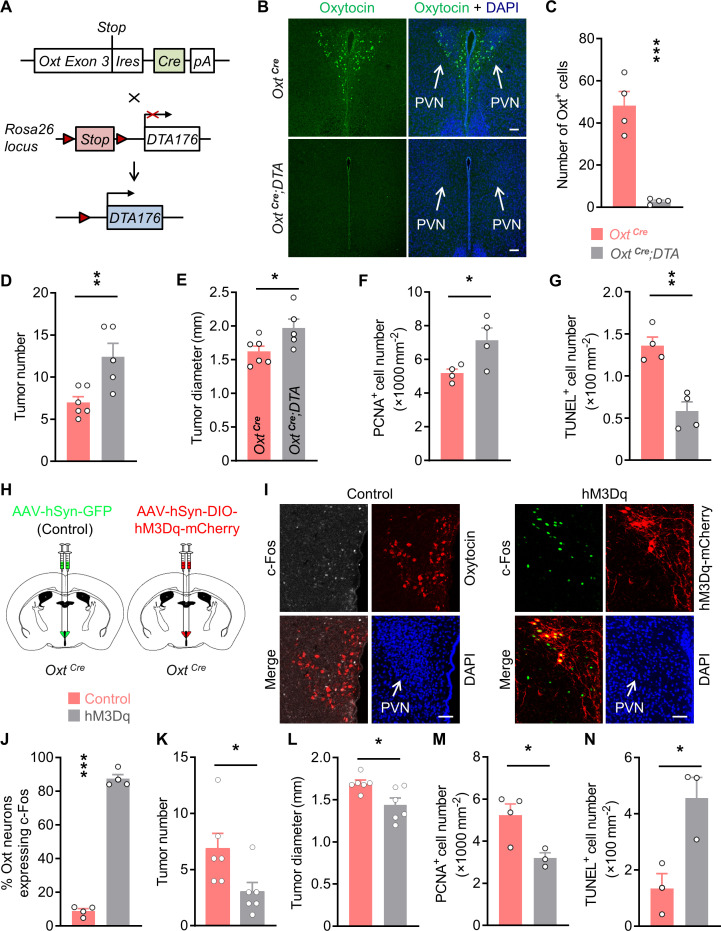
Figure 1. Oxytocin (Oxt) neurons modulate the progression of azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced colitis-associated cancer (CAC) in mice.
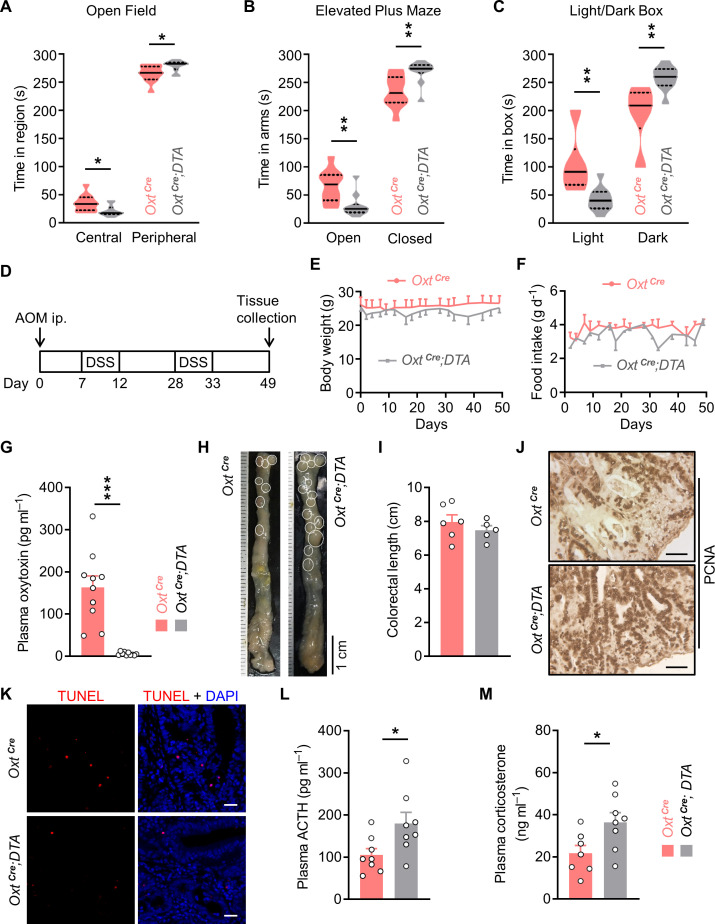
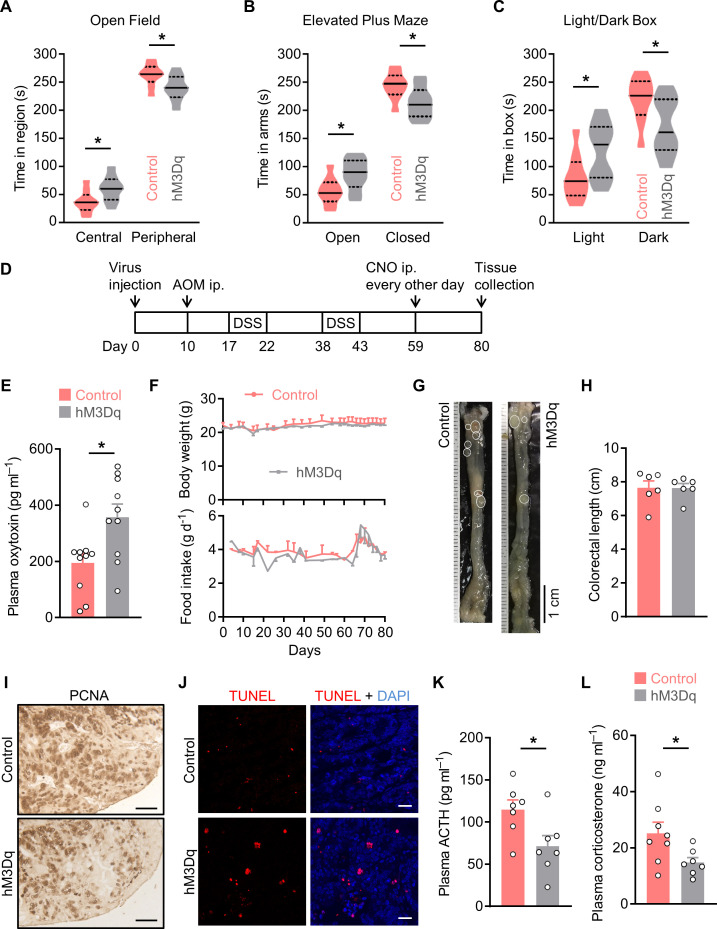
(A) A schematic diagram showing the strategy of generating OxtCre;DTA mice. When Cre recombinase is present, loxP-flanked Stop cassette is excised, therefore allowing the expression of DTA176 in Oxt neurons. Triangles represent loxP sites. Ires, internal ribosome entry site. pA, simian virus 40 polyadenylation signal. (B) The CAC was induced in the 2-month-old OxtCre and OxtCre;DTA mice using AOM and DSS (see also Figure 1—figure supplement 1D). After completing the experiment, immunofluorescent staining for Oxt (green) indicated that Oxt neurons had been depleted in the paraventricular nucleus (PVN) of OxtCre;DTA mice. Cell nuclei were counterstained with DAPI (blue). Scale bars, 100 μm. (C) The number of Oxt-positive cells in the PVN. n = 4 mice per group. (D and E) The CAC was induced in the 2-month-old OxtCre and OxtCre;DTA mice using AOM and DSS. Tumor number (D) and diameter (E) in mice treated with AOM/DSS are shown. n = 6 (OxtCre) or 5 (OxtCre;DTA) mice per group. (F) The density of proliferating cell nuclear antigen (PCNA)-positive cells in the tumor tissues of AOM/DSS-treated OxtCre and OxtCre;DTA mice. n = 4 mice per group. (G) The density of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL)-positive cells in tumor tissues. n = 4 mice per group. (H) Schematic diagrams showing that the indicated adeno-associated viruses (AAVs) were injected into mouse PVN. (I) Adult male OxtCre mice were injected with AAV-hSyn-GFP (control) or AAV-hSyn-DIO-hM3Dq-mCherry (hM3Dq) viruses into the PVN, and were then administered with AOM and DSS. The mice were i.p. injected with clozapine-N-oxide (CNO) every other day for 3 weeks (see also Figure 1—figure supplement 2D). Two hours after the final dose of CNO, mice were perfused with 4% paraformaldehyde (PFA). For control, we carried out double immunofluorescence staining for c-Fos (gray) and Oxt (red). For hM3Dq, immunostaining for c-Fos (green) was performed, and Oxt neurons were identified using hM3Dq-mCherry (red). DAPI staining is in blue. Scale bars, 50 μm. (J) The percentage of OxtPVN neurons expressing c-Fos. n = 4 mice per group. (K and L) Male OxtCre mice (2 months of age) were injected with the indicated AAV into PVN, and were then treated with AOM and DSS. Subsequently, mice were i.p. administered with CNO every other day for 3 weeks. The animals were then sacrificed and tumor number (K) as well as diameter (L) were assessed. n = 6 mice per group. (M) The density of PCNA-positive cells in tumor tissues. n = 4 (control) or 3 (hM3Dq) mice. (N) The density of TUNEL-positive cells in tumor tissues. n = 3 mice per group. Data are shown as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, two-tailed Student’s t-test (C–G, J–N).