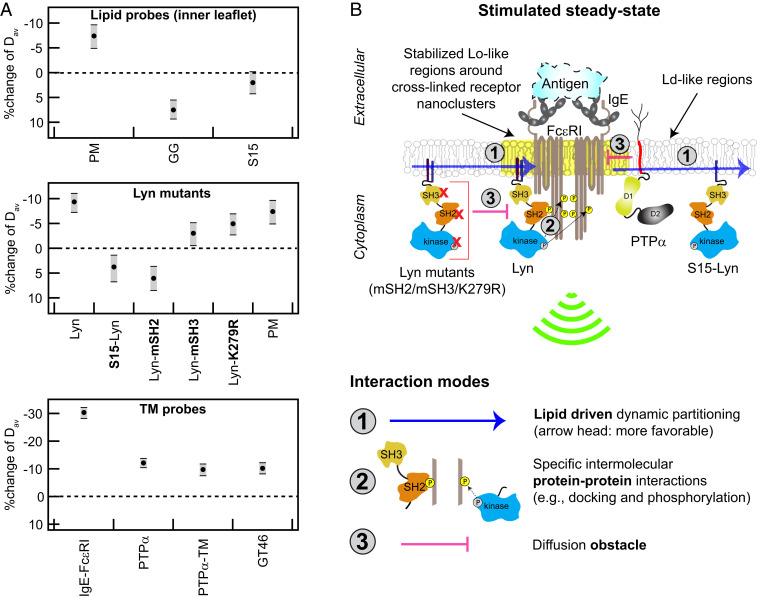
Fig. 7.
Ag-crosslinking of IgE-FcεRI stabilizes surrounding Lo-like nanodomains, causing dynamic lipid- and protein-based interactions that shift diffusion properties of signaling components and lead to suprathreshold phosphorylation by Lyn. (A) Stimulated changes in Dav for inner leaflet lipid probes, Lyn variants, and TM probes, including IgE-FcεRI and PTPα. Values and error bars represent effect change distributions shown in Figs. 2–6. (B) Proposed interaction modes leading to functional coupling of Lyn with clustered FcεRI: Stabilized Lo-like environment preferentially includes Lo-preferring Lyn and excludes Ld-preferring S15-Lyn and PTPα (interaction mode 1). Preferentially proximal Lyn (interaction mode 1) phosphorylates clustered FcεRI via its kinase module and then binds to pTyr via its SH2 module as facilitated by its SH3 module (interaction mode 2); these cumulative interactions stabilize the coupling. Lyn variants with impaired kinase, SH2, or SH3 modules are sterically hindered by cytoplasmic segments of clustered FcεRI (interaction mode 3). PTPα, which is preferentially excluded from Lo-like environments (interaction mode 1), is further limited in access to FcεRI-pTyr due to steric hindrance by clustered FcεRITMDs (interaction mode 3).