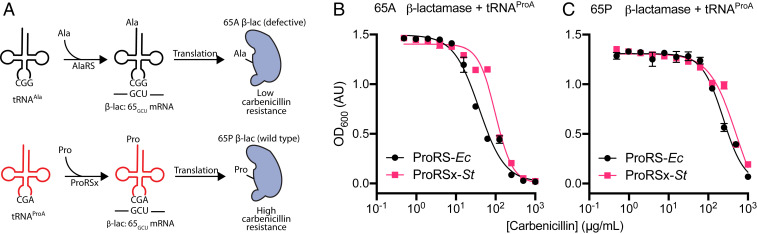
Fig. 4.
The S. turgidiscabies ProRSx/tRNAProA pair appears to cause mistranslation of alanine codons when expressed in E. coli. (A) A β-lactamase–based assay was used to monitor the mistranslation of alanine codons with proline in E. coli. The assay is based on the fact that the β-lactamase mutant Pro65Ala (β-lac 65A) has poor activity and does not protect E. coli cells against the antibiotic carbenicillin. However, when cells mistranslate alanine codons as proline, this should restore β-lactamase activity and increase the resistance of E. coli to carbenicillin. (B and C) Measurements of E. coli growth in the presence of carbenicillin. (B) E. coli cells harboring β-lactamase mutant (β-lac 65A) and expressing ProRSx-St and tRNAProA tolerate higher doses of carbenicillin, relative to cells expressing ProRS-Ec and tRNAProA. The IC50 values of both datasets are different as estimated by an extra sum-of-squares F test with a P value < 0.0001. (C) Expression of ProRSx-St or ProRS-Ec did not increase carbenicillin resistance in E. coli cells expressing the WT β-lactamase (β-lac 65P) and tRNAProA. Each point represents the average of four biological replicates with the SD indicated.