Figure 6.
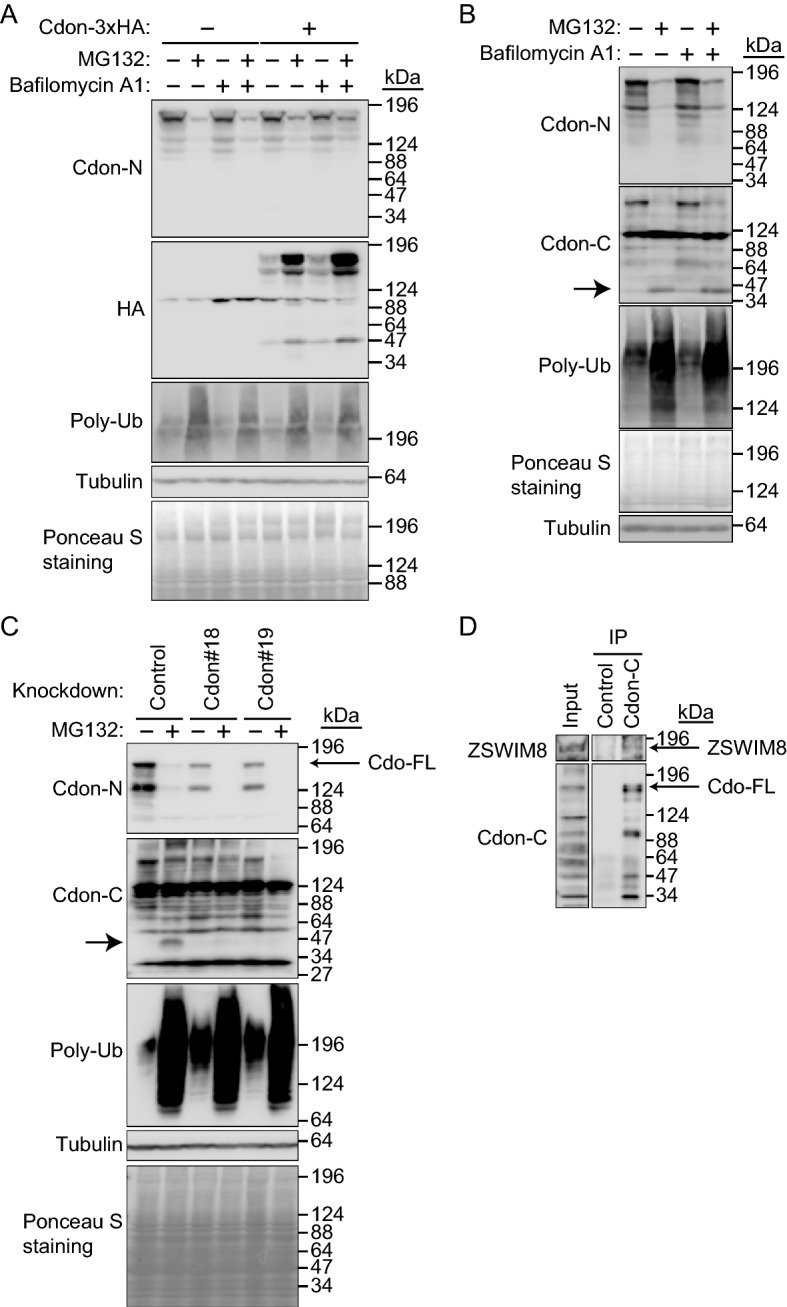
Downregulation of full-length Cdon by the proteasome inhibitor MG132 but not by the lysosome inhibitor bafilomycin A1. (A) Downregulation and accumulation of endogenous Cdon and Cdon-3HA, respectively, with the proteasome inhibitor MG132. Cdon-3 × HA was transiently expressed in C2C12 cells and cultured with or without MG132 (10 μM) and/or bafilomycin A1 (0.5 μM) for 7 h. The cell lysates were subjected to immunoblotting with an anti-Cdon-N, HA, or polyubiquitin (Poly-Ub) antibody. Tubulin and Ponceau S staining were used as loading controls. Representative data of three independent experiments. (B) Accumulation of Cdon fragment with MG132. C2C12 cells were cultured with or without MG132 (10 μM) and/or bafilomycin A1 (0.5 μM) for 7 h. The cell lysates were subjected to immunoblotting with an anti-Cdon-N, Cdon-C, or Poly-Ub antibody. Tubulin and Ponceau S staining were used as loading controls. The MG132-dependent Cdon fragment is indicated by an arrow. Representative data of three independent experiments. (C) Downregulation of Cdon fragment by Cdon knockdown. Control or Cdon-knockdown (#18 or 19) C2C12 cells were cultured with or without MG132 (10 μM) for 7 h. The cell lysates were subjected to immunoblotting with an anti-Cdon-N, Cdon-C, Poly-Ub, or Tubulin antibody. Tubulin and Ponceau S staining were used as loading controls. The MG132-dependent Cdon fragment is indicated by an arrow. Representative data of three independent experiments. (D) Endogenous interaction between ZSWIM8 and Cdon. C2C12 cells were differentiated for 4 days. The cell lysates were subjected to immunoprecipitation with an anti-Cdon-C or control antibody and immunoblotted with an anti-ZSWIM8 or Cdon-C antibody. Representative data of two independent experiments. The membranes in (A) to (D) were cut prior to hybridization with antibodies. Full-length blots are presented in Supplementary Fig. 10.
