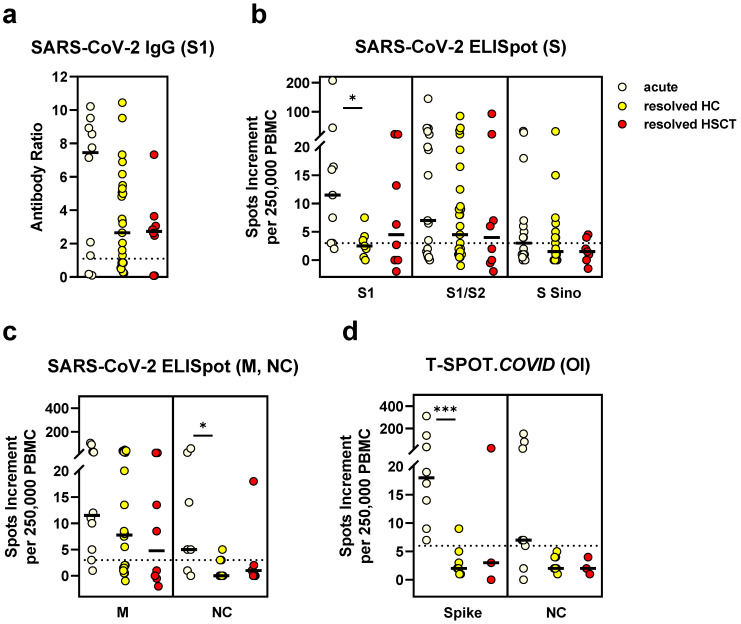
Figure 4.
SARS-CoV-2-specific IgG and ELISpot responses in hematopoietic stem cell transplant (HSCT) recipients and non-immunosuppressed controls after SARS-CoV-2 infection. We compared the data in HSCT patients (resolved HSCT, n = 8) with those of controls with acute SARS-CoV-2 infection (n = 17) or with resolved SARS-CoV-2 infection (resolved HC, n = 27). Panel (a) shows IgG antibody responses against SARS-CoV-2 spike (S) 1, (b) ELISpot responses to S antigens (S) and (c) to membrane (M) and nucleocapsid (NC). For comparison with these in house ELISpot assays (b,c), results of the T-SPOT.COVID (Oxford Immunotec, OI) are shown as panel (d). Parallel tests with both ELISpot formats were performed in 19 volunteers. Horizontal bold lines indicate median values, and dashed lines the cutoff for positive responses (antibody ratio of 1.1, 3 spots increment for in house ELISpot assays and 6 spots increment for the T-SPOT.COVID). S1-peptide mix of the SARS-CoV-2 spike (S) 1; S1/S2-peptide mix of the spike (S) 1 and S2; S Sino-S1 protein (Sino Biological); M-peptide mix of the membrane; NC-peptide mix of the nucleocapsid. * p < 0.05, *** p < 0.001 (Mann–Whitney test for controls with acute vs. resolved infection).