Abstract
SARC-F is a screening tool for sarcopenia; however, it has not yet been established whether SARC-F scores predict functional outcomes. Therefore, we herein investigated the relationship between SARC-F scores and functional outcomes in stroke patients. The primary outcome in the present study was the modified Rankin Scale (mRS) 3 months after stroke. The relationship between SARC-F scores and poor functional outcomes was examined using a logistic regression analysis. Furthermore, the applicability of SARC-F scores to the assessment of poor functional outcomes was analyzed based on the area under the receiver operating curve (ROC). Eighty-one out of the 324 patients enrolled in the present study (25%) had poor functional outcomes (mRS ≥ 4). The results of the multivariate analysis revealed a correlation between SARC-F scores (OR = 1.29, 95% CI = 1.05–1.59, p = 0.02) and poor functional outcomes. A cut-off SARC-F score ≥ 4 had low-to-moderate sensitivity (47.4%) and high specificity (87.3%). The present results suggest that the measurement of pre-stroke SARC-F scores is useful for predicting the outcomes of stroke patients.
Keywords: stroke, sarcopenia, SARC-F score, disability, malnutrition risks
1. Introduction
Stroke is one of the leading causes of disability [1], and the primary cause of disability in stroke patients is brain injury [2]. Stroke increases the risk of sarcopenia [3], which is a contributing factor to disability in these patients [4]. The prevalence of stroke-related sarcopenia increases in the elderly and is associated with the poor recovery of activities of daily living [5,6]. Although sarcopenia may develop in stroke patients, it may also be present pre-stroke, and has been implicated in poor functional outcomes [7,8,9].
SARC-F is a screening tool for sarcopenia in elderly subjects [9,10,11,12] and predicts the risk of pre-stroke sarcopenia [9]. A previous study reported that the risk of developing sarcopenia was elevated in patients with a SARC-F score ≥4 [13]. Since SARC-F has low sensitivity, but high specificity [11,14], the risk of sarcopenia assessed by SARC-F may be underestimated [11,14,15,16]. Another cut-off score was recently reported for predicting the risk of sarcopenia (SARC-F = 1) [17] and the usefulness of SARC-F for detecting the risk of frailty or falls has been demonstrated [18,19]. A previous study revealed a relationship between SARC-F scores and muscle mass [20,21]. Based on these findings, SARC-F scores may be useful for predicting the risk of pre-stroke sarcopenia and functional outcomes in stroke patients.
The aim of the present study was to investigate the relationship between pre-stroke SARC-F scores and functional outcomes in stroke patients. We hypothesized that SARC-F scores are associated with functional outcomes, even after adjustments for confounding factors, such as pre-stroke disability or a risk of malnutrition.
2. Materials and Methods
2.1. Study Design and Participants
This prospective cohort study was conducted between August 2017 and December 2019 and included elderly patients consecutively admitted to Itami Kousei Neurosurgical Hospital within 48 h of stroke onset. The following patients were enrolled: age ≥ 65 years and evidence of cerebral infarction or intracerebral hemorrhage on computed tomography or magnetic resonance imaging. Exclusion criteria were (1) pre-stroke dependent ambulation, (2) patients unable to complete the questionnaire because of impaired consciousness, cognitive dysfunction, or language disorders, such as aphasia, and (3) the lack of informed consent. The present study was approved by the Research Ethics Committee of Konan Women’s University, and all patients provided their informed consent.
2.2. SARC-F
The SARC-F questionnaire, which evaluates the pre-stroke status and has been adapted for a Japanese population, was completed by patients within 5 days of admission. It comprises the following components: strength, assistance with walking, rising from a chair, climbing stairs, and falls [12]. SARC-F scores range between 0 and 10 (0 = best, 10 = worst), with each component receiving 0–2 points.
2.3. Assessment of a Risk of Malnutrition and Comorbidities
The Geriatric Nutritional Risk Index (GNRI) was employed to evaluate the nutritional status of patients [22], and was calculated as follows: GNRI = (1.489 × serum albumin [g/dL]) + 103 (41.7 × weight [kg]/ideal body weight). In cases in which weight/ideal body weight was ≥1.0, the ratio was set to 1. GNRI ≤ 98 kg/m2 was defined as a risk of malnutrition as previously reported [22].
2.4. Clinical Characteristics
Information was obtained from electronic medical records on the following patient characteristics: age, sex, height, body weight, body mass index, neurological deficits evaluated by the National Institutes of Health Stroke Scale (NIHSS) score, stroke type, lesion laterality, and pre-stroke mRS. Pre-stroke disability was defined as mRS = 2 (slight disability) or 3 (moderate disability), and no disability as mRS = 0 (no symptoms) or 1 (no significant disability).
2.5. Main Outcome
The primary outcome of the present study was the modified Rankin Scale (mRS) evaluated 3 months after stroke from medical records or in a telephone interview. mRS was scored based on an unstructured direct interview by a physician [23], with 0 = no symptoms, 1 = no significant disability despite the presence of symptoms; capable of performing all of the usual duties and activities, 2 = slight disability; unable to perform all of the previous activities, but capable of attending to one’s own affairs without assistance, 3 = moderate disability; requiring some help, but capable of walking without assistance, 4 = moderately severe disability; unable to walk or attend to one’s own bodily needs without assistance, 5 = severe disability; bedridden, incontinent, and requiring constant nursing care and attention, and 6 = dead [24]. A poor outcome was defined as mRS scores 3 months after stroke of 4–6 [25].
2.6. Statistical Analysis
Data are shown as medians (interquartile range; IQR) and numbers (%) for categorical data. The unadjusted and adjusted odds ratios (OR) of SARC-F scores and poor functional outcomes (mRS ≥ 4) were calculated by a logistic regression analysis. Confounding factors were adjusted for, including age [26], sex [27], NIHSS [28], pre-stroke disability [29], and malnutrition risk [22,30]. The applicability of SARC-F scores to evaluations of poor functional outcomes was examined in an analysis of the area under the receiver operating curve (ROC). Sensitivity and 1-specificity were calculated from the obtained sensitivity and specificity, and the point at the maximal value was taken as the optimum cut-off value. The area under the curve, sensitivity, specificity, and positive and negative predictive values for SARC-F were analyzed. All statistical analyses were performed using SPSS version 20.0 (SPSS, Inc., Chicago, IL, USA). Differences with p < 0.05 were considered to be significant.
3. Results
During the study period, 643 elderly stroke patients were hospitalized, and 293 were excluded from the analysis due to admission 48 h after stroke symptom onset (n = 17), pre-stroke dependent ambulation (n = 74), impaired consciousness (n = 68), cognitive dysfunction (n = 64), and aphasia (n = 39). Ten patients also refused to participate and 21 did not provide informed consent. Among the 350 patients enrolled, 26 patients refused a follow-up. Therefore, 324 stroke patients were included in the present study.
Among the patients enrolled, 54 (17%) had pre-stroke disability and 41 (13%) were at risk of malnutrition. Eighty-one patients (25%) had poor functional outcomes (mRS ≥ 4) 3 months after stroke (Table 1). Table 2 shows the distribution for each SARC-F score in all patients. Approximately 50% of patients had an SARC-F score of zero.
Table 1.
Stroke patient characteristics.
| Total Cohort | |
|---|---|
| (n = 324) | |
| Age (years, median (IQR)) | 76 (11) |
| Sex (male/female) | 187/137 |
| Body mass index (kg/m2, median [IQR]) | 22.5 (4.2) |
| NIHSS (median [IQR]) | 2 (3) |
| Stroke type (infarction/hemorrhage) | 267/57 |
| Lesion side (right/left/both) | 161/152/11 |
| Pre-stroke disability (%) | 54 (17) |
| Stroke risk factors (%) | |
| Hypertension | 159 (49) |
| Diabetes | 74 (23) |
| Previous stroke | 89 (28) |
| Hypercholesterolemia | 78 (24) |
| Ischemic heart disease | 27 (8) |
| Atrial fibrillation | 27 (8) |
| Smoking | 110 (34) |
| Risk of malnutrition (%) | 41 (13) |
| mRS 3 months after stroke (%) | |
| 0 | 31 (10) |
| 1 | 105 (32) |
| 2 | 58 (18) |
| 3 | 49 (15) |
| 4 | 69 (21) |
| 5 | 10 (3) |
| 6 | 2 (1) |
| Poor functional outcome (%) | 81 (25) |
IQR = interquartile range; NIHSS = National Institutes of Health Stroke Scale; mRS = modified Rankin Scale.
Table 2.
SARC-F scores and their distribution.
| Total Cohort | |
|---|---|
| (n = 324) | |
| SARC-F score (median [IQR]) | 1 (3) |
| SARC-F score distribution | |
| score = 0 (%) | 158 (49) |
| score = 1 (%) | 44 (13) |
| score = 2 (%) | 31 (10) |
| score = 3 (%) | 30 (9) |
| score = 4 (%) | 30 (9) |
| score = 5 (%) | 14 (4) |
| score = 6 (%) | 2 (1) |
| score = 7 (%) | 5 (2) |
| score = 8 (%) | 7 (2) |
| score = 9 (%) | 3 (1) |
| score = 10 (%) | 0 (0) |
IQR = interquartile range.
Based on unadjusted OR, age (OR = 1.06, 95% confidence interval (CI) = 1.02–1.10, p = 0.007), NIHSS (OR = 1.40, 95% CI = 1.36–1.68, p < 0.001), pre-stroke disability (OR = 5.75, 95% CI = 3.00–10.99, p < 0.001), risk of malnutrition (OR = 3.78, 95% CI = 1.86–7.68, p < 0.001), and SARC-F scores (OR = 1.44, 95% CI = 1.27–1.64, p < 0.001) correlated with poor functional outcomes 3 months after stroke.
After adjustments for these factors, NIHSS (OR = 1.56, 95% CI = 1.39–1.76, p < 0.001), pre-stroke disability (OR = 3.22, 95% CI = 1.11–9.34, p = 0.03), and SARC-F scores (OR = 1.29, 95% CI = 1.05–1.59, p = 0.02) correlated with poor functional outcomes (Table 3).
Table 3.
Logistic regression analysis of poor functional outcomes.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| Odds Ratio | p-Value | Odds Ratio | p-Value | |
| (95% CI) | (95% CI) | |||
| Age | 1.06 | 0.007 | 1.03 | 0.41 |
| (1.02–1.10) | (0.97–1.09) | |||
| Sex | 1.4 | 0.25 | 0.73 | 0.46 |
| (0.79–2.48) | (0.31–1.69) | |||
| NIHSS | 1.51 | <0.001 | 1.56 | <0.001 |
| (1.36–1.68) | (1.39–1.76) | |||
| Pre-stroke disability | 5.75 | <0.001 | 3.22 | 0.03 |
| (3.00–10.99) | (1.11–9.34) | |||
| Risk of malnutrition | 3.78 | <0.001 | 2.5 | 0.08 |
| (1.86–7.68) | (0.89–7.06) | |||
| SARC-F score | 1.44 | <0.001 | 1.29 | 0.02 |
| (1.27–1.64) | (1.05–1.59) | |||
CI = confidence interval; NIHSS = National Institutes of Health Stroke Scale.
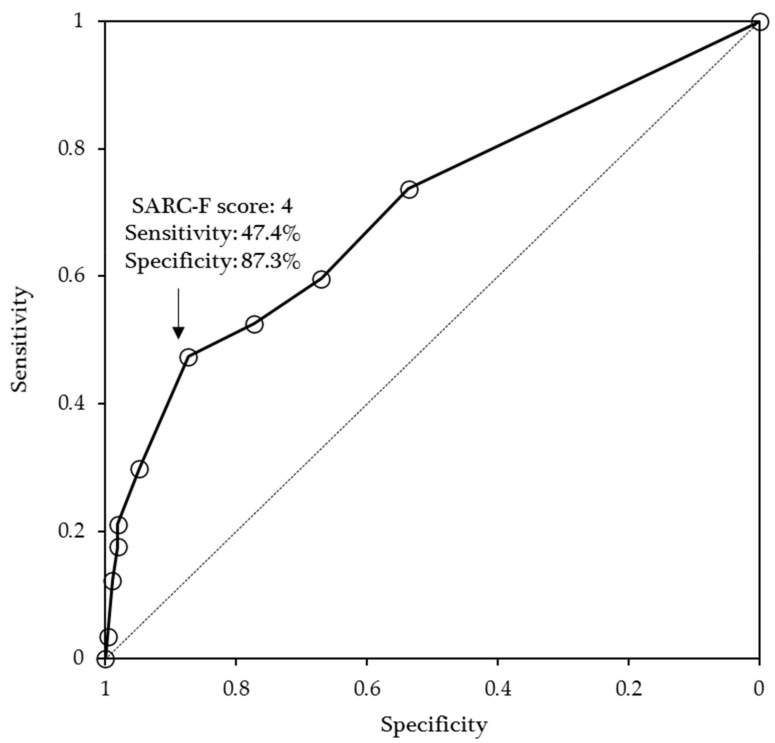
The results of the ROC curve analysis are shown in Figure 1. An SARC-F cut-off ≥4 had low to moderate sensitivity (47.4%) and high specificity (87.3%) to screen for poor functional outcomes (positive predictive value = 44.3%, negative predictive value = 88.6%, AUC = 0.702, 95% Cl = 0.620–0.784).
Figure 1.
Receiver operating characteristic curves of SARC-F scores as an indicator of poor outcomes.
4. Discussion
This prospective cohort study examined the relationship between functional outcomes and pre-stroke SARC-F scores in stroke patients. The results obtained revealed the independent effects of pre-stroke SARC-F scores on functional outcomes and also supported the applicability of an SARC-F score ≥ 4 for predicting poor functional outcomes in stroke patients.
Previous studies reported a relationship between pre-stroke sarcopenia and poor outcomes [7,8,9]; however, these studies did not consider pre-stroke disability as a confounding factor. Sarcopenia is generally associated with disability [31,32,33], and pre-stroke disability has a negative impact on functional outcomes in stroke patients [29,34]. Therefore, the effects of pre-stroke sarcopenia on physical function need to be interpreted in consideration of pre-stroke disability. If a patient responds “unable”, the results of SARC-F also need to be considered as the result of an assessment of disability. However, the present results demonstrated the independent effects of SARC-F scores on poor functional outcomes even after adjustments for pre-stroke disability. Therefore, pre-stroke SARC-F scores are useful not only for sarcopenia screening, but also for predicting poor functional outcomes in stroke patients.
The cut-off SARC-F score for sarcopenia screening is ≥4; however, a previous study demonstrated the low sensitivity and high specificity of this score for detecting sarcopenia [11,14]. We also investigated whether a cut-off SARC-F score ≥ 4 had low to moderate sensitivity and high specificity for the screening of poor functional outcomes in stroke patients. A SARC-F score ≥ 4 was previously associated with poor functional outcomes; however, this cut-off value was not validated [9]. The present results confirmed the validity of a SARC-F score ≥ 4 in stroke patients.
There are a number of limitations that need to be addressed. Younger patients or those with consciousness disorders, severe cognitive dysfunction, or aphasia were excluded from the present study. Since severe stroke patients were not enrolled, the present results may not be applicable to all stroke patients. Furthermore, the present study was conducted in a small, single-center setting and did not adjust for many confounding factors. Another limitation is that the impact of SARC-F scores on mortality or long-term outcomes was not examined. Therefore, further multicenter, large-scale, and long-term studies are needed to obtain more general and useful results for these patients.
5. Conclusions
Pre-stroke SARC-F scores were associated with poor functional outcomes in stroke patients even after adjustments for confounding factors, such as pre-stroke disability or a risk of malnutrition, and a SARC-F score ≥ 4 was suitable for predicting poor outcomes in these patients. Assessments of the pre-stroke status using SARC-F scores may be useful for predicting the outcomes of stroke patients.
Acknowledgments
We thank the individuals who participated in this study and the physical therapy staff of the Department of Rehabilitation, Itami Kousei Neurosurgical Hospital for their assistance.
Author Contributions
Conceptualization, M.N., H.K., M.Y. and M.K.; methodology, M.N.; formal analysis, M.N.; investigation, M.N., H.K., M.Y. and M.K.; data curation, M.N.; writing—original draft preparation, M.N.; writing—review and editing, H.K., M.Y. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a research grant from JSPS KAKENHI (grant number 21K11182).
Institutional Review Board Statement
The present study was performed according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of Konan Women’s University (protocol code 2015020 and date of approval April 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the present study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author following permission by the Ethics Committee and the hospital at which the study was conducted.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hardie K., Hankey G.J., Jamrozik K., Broadhurst R.J., Anderson C. Ten-Year Risk of First Recurrent Stroke and Disability after First-Ever Stroke in the Perth Community Stroke Study. Stroke. 2004;35:731–735. doi: 10.1161/01.STR.0000116183.50167.D9. [DOI] [PubMed] [Google Scholar]
- 2.Luft A.R., Forrester L., Macko R.F., McCombe-Waller S., Whitall J., Villagra F., Hanley D.F. Brain Activation of Lower Extremity Movement in Chronically Impaired Stroke Survivors. NeuroImage. 2005;26:184–194. doi: 10.1016/j.neuroimage.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Ryan A.S., Ivey F.M., Serra M.C., Hartstein J., Hafer-Macko C.E. Sarcopenia and Physical Function in Middle-Aged and Older Stroke Survivors. Arch. Phys. Med. Rehabil. 2017;98:495–499. doi: 10.1016/j.apmr.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scherbakov N., von Haehling S., Anker S.D., Dirnagl U., Doehner W. Stroke Induced Sarcopenia: Muscle Wasting and Disability after Stroke. Int. J. Cardiol. 2013;170:89–94. doi: 10.1016/j.ijcard.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Yoshimura Y., Wakabayashi H., Bise T., Tanoue M. Prevalence of Sarcopenia and Its Association with Activities of Daily Living and Dysphagia in Convalescent Rehabilitation Ward Inpatients. Clin. Nutr. 2018;37:2022–2028. doi: 10.1016/j.clnu.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura Y., Wakabayashi H., Bise T., Nagano F., Shimazu S., Shiraishi A., Yamaga M., Koga H. Sarcopenia is Associated with Worse Recovery of Physical Function and Dysphagia and a Lower Rate of Home Discharge in Japanese Hospitalized Adults Undergoing Convalescent Rehabilitation. Nutrition. 2019;61:111–118. doi: 10.1016/j.nut.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Ohyama K., Watanabe M., Nosaki Y., Hara T., Iwai K., Mokuno K. Correlation Between Skeletal Muscle Mass Deficit and Poor Functional Outcome in Patients with Acute Ischemic Stroke. J. Stroke Cerebrovasc. Dis. 2020;29:104623. doi: 10.1016/j.jstrokecerebrovasdis.2019.104623. [DOI] [PubMed] [Google Scholar]
- 8.Abe T., Iwata K., Yoshimura Y., Shinoda T., Inagaki Y., Ohya S., Yamada K., Oyanagi K., Maekawa Y., Honda A., et al. Low Muscle Mass is Associated with Walking Function in Patients with Acute Ischemic Stroke. J. Stroke Cereb. Dis. 2020;29:105259. doi: 10.1016/j.jstrokecerebrovasdis.2020.105259. [DOI] [PubMed] [Google Scholar]
- 9.Nozoe M., Kanai M., Kubo H., Yamamoto M., Shimada S., Mase K. Prestroke Sarcopenia and Functional Outcomes in Elderly Patients Who Have Had an Acute Stroke: A Prospective Cohort Study. Nutrition. 2019;66:44–47. doi: 10.1016/j.nut.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Malmstrom T.K., Miller D.K., Simonsick E.M., Ferrucci L., Morley J.E. SARC-F: A Symptom Score To Predict Persons with Sarcopenia at Risk for Poor Functional Outcomes. J. Cachexia-Sarcopenia Muscle. 2016;7:28–36. doi: 10.1002/jcsm.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ida S., Kaneko R., Murata K. SARC-F for Screening of Sarcopenia among Older Adults: A Meta-Analysis of Screening Test Accuracy. J. Am. Med. Dir. Assoc. 2018;19:685–689. doi: 10.1016/j.jamda.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka S., Kamiya K., Hamazaki N., Matsuzawa R., Nozaki K., Maekawa E., Noda C., Yamaoka-Tojo M., Matsunaga A., Masuda T., et al. Utility of SARC-F for Assessing Physical Function in Elderly Patients with Cardiovascular Disease. J. Am. Med. Dir. Assoc. 2017;18:176–181. doi: 10.1016/j.jamda.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Chen L.-K., Woo J., Assantachai P., Auyeung T.-W., Chou M.-Y., Iijima K., Jang H.C., Kang L., Kim M., Kim S., et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020;21:300–307. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Lu J.-L., Ding L.-Y., Xu Q., Zhu S.-Q., Xu X.-Y., Hua H.-X., Chen L., Xu H. Screening Accuracy of SARC-F for Sarcopenia in the Elderly: A Diagnostic Meta-Analysis. J. Nutr. Health Aging. 2021;25:172–182. doi: 10.1007/s12603-020-1471-8. [DOI] [PubMed] [Google Scholar]
- 15.Ishida Y., Maeda K., Nonogaki T., Shimizu A., Yamanaka Y., Matsuyama R., Kato R., Ueshima J., Murotani K., Mori N. SARC-F as a Screening Tool for Sarcopenia and Possible Sarcopenia Proposed by AWGS 2019 in Hospitalized Older Adults. J. Nutr. Health Aging. 2020;24:1053–1060. doi: 10.1007/s12603-020-1462-9. [DOI] [PubMed] [Google Scholar]
- 16.Kera T., Kawai H., Hirano H., Kojima M., Watanabe Y., Motokawa K., Fujiwara Y., Osuka Y., Kojima N., Kim H., et al. Limitations of SARC-F in the Diagnosis of Sarcopenia in Community-Dwelling Older Adults. Arch. Gerontol. Geriatr. 2020;87:103959. doi: 10.1016/j.archger.2019.103959. [DOI] [PubMed] [Google Scholar]
- 17.Erbas Sacar D., Kilic C., Karan M.A., Bahat G. Ability of SARC-F to Find Probable Sarcopenia Cases in Older Adults. J. Nutr. Health Aging. 2021;25:757–761. doi: 10.1007/s12603-021-1617-3. [DOI] [PubMed] [Google Scholar]
- 18.Bahat G., Ozkok S., Kilic C., Karan M.A. SARC-F Questionnaire Detects Frailty in Older Adults. J. Nutr. Health Aging. 2021;25:448–453. doi: 10.1007/s12603-020-1543-9. [DOI] [PubMed] [Google Scholar]
- 19.Ishida Y., Maeda K., Ueshima J., Shimizu A., Nonogaki T., Kato R., Matsuyama R., Yamanaka Y., Mori N. The SARC-F Score on Admission Predicts Falls during Hospitalization in Older Adults. J. Nutr. Health Aging. 2021;25:399–404. doi: 10.1007/s12603-021-1597-3. [DOI] [PubMed] [Google Scholar]
- 20.Marini A.C.B., Perez D.R.S., Fleuri J.A., Pimentel G.D. SARC-F Is Better Correlated with Muscle Function Indicators than Muscle Mass in Older Hemodialysis Patients. J. Nutr. Health Aging. 2020;24:999–1002. doi: 10.1007/s12603-020-1510-5. [DOI] [PubMed] [Google Scholar]
- 21.Siqueira J.M., de Oliveira I.C., Soares J.D., Pimentel G.D., Pimentel G.D. SARC-F Has Low Correlation and Reliability with Skeletal Muscle Mass Index in Older Gastrointestinal Cancer Patients. Clin. Nutr. 2021;40:890–894. doi: 10.1016/j.clnu.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Kang M.K., Kim T.J., Kim Y., Nam K.-W., Jeong H.-Y., Kim S.K., Lee J.S., Ko S.-B., Yoon B.-W. Geriatric Nutritional Risk Index Predicts Poor Outcomes in Patients with Acute Ischemic Stroke-Automated Undernutrition Screen Tool. PLoS ONE. 2020;15:e0228738. doi: 10.1371/journal.pone.0228738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fearon P., McArthur K.S., Garrity K., Graham L.J., McGroarty G., Vincent S., Quinn T.J. Prestroke Modified Rankin Stroke Scale Has Moderate Interobserver Reliability and Validity in an Acute Stroke Setting. Stroke. 2012;43:3184–3188. doi: 10.1161/STROKEAHA.112.670422. [DOI] [PubMed] [Google Scholar]
- 24.Weisscher N., Vermeulen M., Roos Y.B., de Haan R.J. What Should Be Defined as Good Outcome in Stroke Trials; A Modified Rankin Score of 0–1 or 0–2? J. Neurol. 2008;255:867–874. doi: 10.1007/s00415-008-0796-8. [DOI] [PubMed] [Google Scholar]
- 25.Rangaraju S., Haussen D.C., Nogueira R.G., Nahab F., Frankel M. Comparison of 3-Month Stroke Disability and Quality of Life across Modified Rankin Scale Categories. Interv. Neurol. 2017;6:36–41. doi: 10.1159/000452634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakayama H., Jørgensen H.S., O Raaschou H., Olsen T.S. The Influence of Age on Stroke Outcome. The Copenhagen Stroke Study. Stroke. 1994;25:808–813. doi: 10.1161/01.STR.25.4.808. [DOI] [PubMed] [Google Scholar]
- 27.Carcel C., Wang X., Sandset E.C., Delcourt C., Arima H., Lindley R., Hackett M.L., Lavados P., Robinson T.G., Muñoz Venturelli P., et al. Sex Differences in Treatment and Outcome after Stroke: Pooled Analysis Including 19,000 Participants. Neurology. 2019;93:2170–2180. doi: 10.1212/WNL.0000000000008615. [DOI] [PubMed] [Google Scholar]
- 28.Sato S., Toyoda K., Uehara T., Toratani N., Yokota C., Moriwaki H., Naritomi H., Minematsu K. Baseline NIH Stroke Scale Score Predicting Outcome in Anterior and Posterior Circulation Strokes. Neurology. 2008;70:2371–2377. doi: 10.1212/01.wnl.0000304346.14354.0b. [DOI] [PubMed] [Google Scholar]
- 29.Ganesh A., Luengo-Fernandez R., Pendlebury S.T., Rothwell P.M. Long-Term Consequences of Worsened Poststroke Status in Patients with Premorbid Disability. Stroke. 2018;49:2430–2436. doi: 10.1161/STROKEAHA.118.022416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis J.P., Wong A.A., Schluter P.J., Henderson R.D., O’Sullivan J.D., Read S.J. Impact of Premorbid Undernutrition on Outcome in Stroke Patients. Stroke. 2004;35:1930–1934. doi: 10.1161/01.STR.0000135227.10451.c9. [DOI] [PubMed] [Google Scholar]
- 31.Phillips A., Strobl R., Vogt S., Ladwig K.-H., Thorand B., Grill E. Sarcopenia Is Associated with Disability Status-Results from the KORA-Age Study. Osteoporos. Int. 2017;28:2069–2079. doi: 10.1007/s00198-017-4027-y. [DOI] [PubMed] [Google Scholar]
- 32.Fang Q., Zhu G., Huang J., Pan S., Fang M., Li Q., Yin Q., Liu X., Tang Q., Huang D., et al. Current Status of Sarcopenia in the Disabled Elderly of Chinese Communities in Shanghai: Based on the Updated EWGSOP Consensus for Sarcopenia. Front. Med. 2020;12:552415. doi: 10.3389/fmed.2020.552415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimada H., Tsutsumimoto K., Doi T., Lee S., Bae S., Nakakubo S., Makino K., Arai H. Effect of Sarcopenia Status on Disability Incidence Among Japanese Older Adults. J. Am. Med. Dir. Assoc. 2021;22:846–852. doi: 10.1016/j.jamda.2020.10.036. [DOI] [PubMed] [Google Scholar]
- 34.Han T.S., Fry C.H., Gulli G., Affley B., Robin J., Irvin-Sellers M., Fluck D., Kakar P., Sharma S., Sharma P. Prestroke Disability Predicts Adverse Poststroke Outcome: A Registry-Based Prospective Cohort Study of Acute Stroke. Stroke. 2020;51:594–600. doi: 10.1161/STROKEAHA.119.027740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author following permission by the Ethics Committee and the hospital at which the study was conducted.