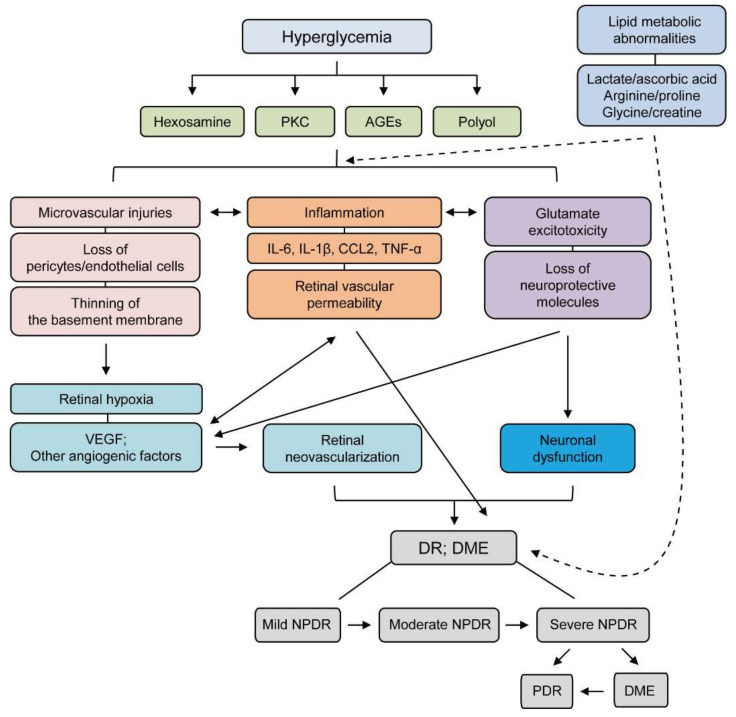
Figure 1.
A schematic illustration of the pathophysiology and stages of diabetic retinopathy (DR). Hyperglycemia evokes various pathological metabolic mechanisms such as accumulation of AGEs and induction of PKC, the polyol, and the hexosamine pathways. Microvascular injuries, inflammation, and glutamate excitotoxicity combine to damage the diabetic retina more severely after induction of these pathways. During these processes, representative features are highlighted: loss of pericytes/endothelial cells, thinning of the basement membrane, increases in IL-6, IL-1β, CCL-2, and TNF-α, retinal vascular permeability, and loss of neuroprotective molecules. These outcomes exacerbate neuronal dysfunction, retinal hypoxia, and increases in various angiogenic factors, including VEGF, which ultimately causes retinal neovascularization. All processes are inter-connected to the development and progression of DR. Furthermore, lipid metabolic abnormalities (changes in levels of lactate, ascorbic acid, arginine, proline, glycine, or creatine) in diabetes could aggravate the intensities of retinal injuries. The stages of DR depending on the severity of the disease: mild NPDR (microaneurysm), moderate NPDR (hemorrhage), severe NPDR (more severe hemorrhage, venous beading, and intraretinal microvascular abnormalities), PDR (new vessel formation, retinal detachment, and vitreous hemorrhage); DME (retinal detachment). Solid line; direct interaction, Dash line; indirect interaction. AGEs; advanced glycation end products, PKC; protein kinase C, CCL; Chemokine (C-C motif) ligand TNF; tumor Necrosis Factor, VEGF; vascular endothelial growth factor, DR; diabetic retinopathy, DME; diabetic macular edema, NPDR; non-proliferative diabetic retinopathy.