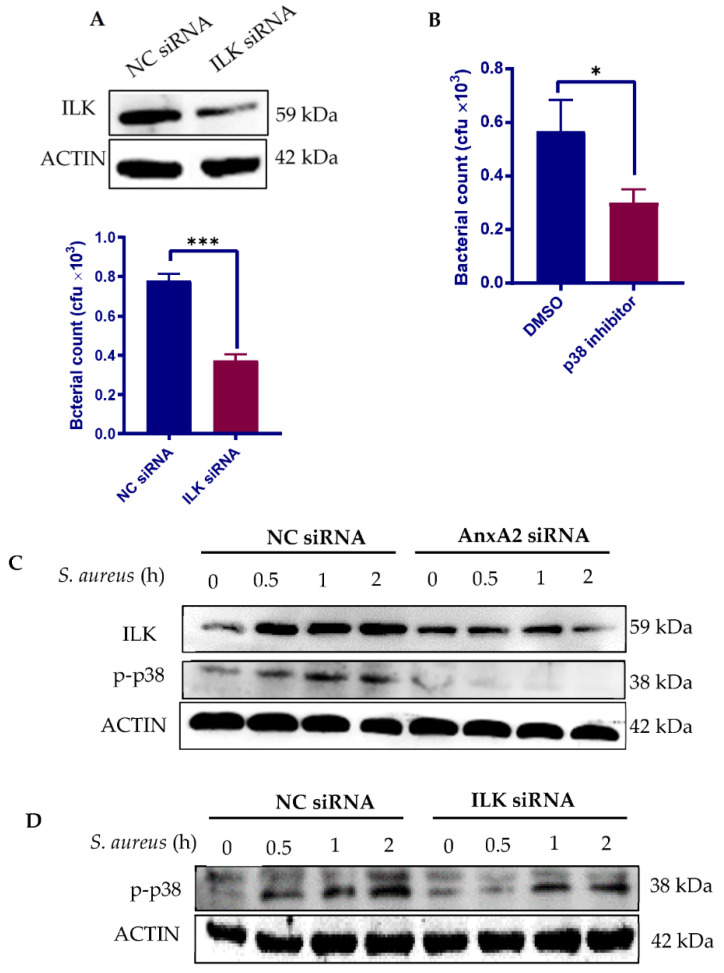
Figure 3.
AnxA2 engagement activates ILK/p38 MAPK pathway. (A) Evaluation of the effect of ILK on S. aureus internalization by MAC-T. MAC-T cells were treated with either NC siRNA or ILK siRNA for 72 h. Whole-cell extracts were analyzed by immunoblotting with anti-ILK (upper panel). β-actin is shown as a loading control. The ILK-deficient cells were then co-cultured with clinical isolate of S. aureus (Clin-SA) at an MOI of 50 for 1 h. Bacterial invasion was determined by gentamicin protection assays (lower panel). The graph shows mean values ± s.d. of three replicates. (B) Evaluation of the effect of p38 MAPK on S. aureus internalization by MAC-T. MAC-T cells were pre-treated with SB203580, a selective inhibitor of p38 MAPK, at 10 μM for 2 h and infected with Clin-SA at an MOI of 50 for 1 h. Bacterial invasion was determined by gentamicin protection assays. The graph shows mean values ± s.d. of three replicates. (C) Evaluation of the effect of AnxA2 engagement on ILK expression and p38 MAPK phosphorylation. MAC-T cells were treated with either NC siRNA or AnxA2 siRNA for 72 h and then subjected to Clin-SA infection at a MOI of 25 for 2 h. Whole-cell extracts were analyzed by immunoblotting with antibodies against AnxA2, ILK and phosphorylated p38 MAPK. β-actin is shown as a loading control. (D) Evaluation of the effect of ILK engagement on p38 MAPK phosphorylation. MAC-T cells were treated with siRNA and Clin-SA as described in (A). Whole-cell extracts were analyzed by immunoblotting with antibodies against ILK and phosphorylated p38 MAPK. β-actin is shown as a loading control. * p < 0.05; *** p < 0.001.