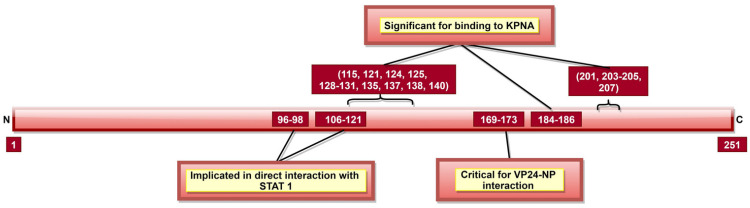
Figure 9.
A schematic representation of EBOV viral protein 24 (VP24). VP24 aa 169–173 facilitate interaction with NP, which is crucial for nucleocapsid (NC) formation, EBOV replication cycle and egress phase. Tyrosine-phosphorylated STAT1, hnRNP C1/C2 and VP24 interact with KPNA at the same position; therefore, upon VP24–KPNA interaction, transport of tyrosine-phosphorylated STAT1 to the nucleus is stopped as well as translocation of hnRNP C1/C2 from the cytoplasm to the nucleus is partially prevented.