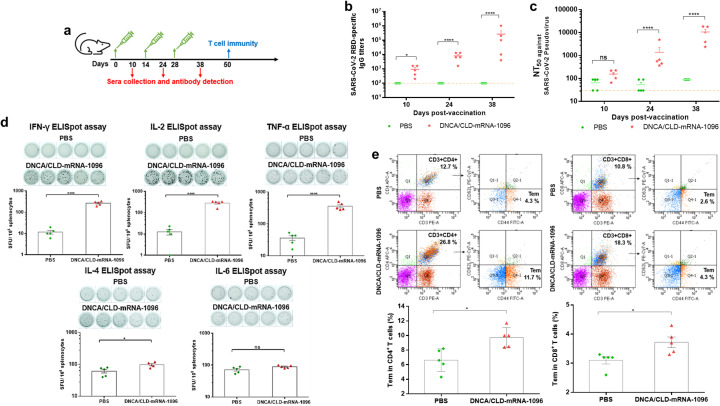
Fig. 5.
The in vivo efficacy and immunogenicity of DNCA/CLD-mRNA-1096. Female BALB/c mice of 6–8 weeks old were vaccinated with DNCA/CLD-mRNA-1096 (mRNA-1096 = 10 μg) or PBS via intramuscular route of administration. (a) The schematic diagram depicted the strategy of immunization, and the plans of sera collection and immunogenicity detection. (b) The SARS-CoV-2 RBD-specific IgG titer in the sera of the vaccinated mice was tested by ELISA. (c) The serum neutralization antibody titer (NT50) was tested against SARS-CoV-2-spike protein pseudovirus. The dashed line showed the limit of the test. (d) ELISpot detection of IFN-γ, IL-2, TNF-α, IL-4, or IL-6 release from the SARS-CoV-2 RBD peptide stimulated splenocytes. (e) Flow cytometric detection of the SARS-CoV-2 RBD-specific CD4+ and CD8+ effector memory T cells (CD44+CD62L−) in splenocytes. Data was shown as means ± SEM. Statistical significance (b-c) was analyzed by a two-way ANOVA analysis followed by Bonferroni's multiple comparisons between the PBS and DNCA/CLD-mRNA-1096 group, (**** p < 0.0001, * p < 0.05; ns: p > 0.05). Statistical significance (d-e) was analyzed by the two-tailed unpaired Student's t-tests, for the comparison of the differences between the PBS and DNCA/CLD-mRNA-1096 group, (**** p < 0.0001, *** P < 0.001, * p < 0.05; ns: p > 0.05).