Fig. 8.
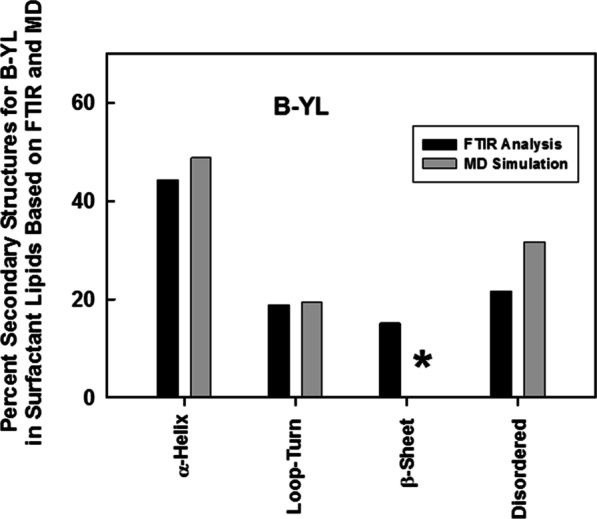
Spectroscopic and MD simulation of the secondary structure for B-YL in lipids. The B-YL peptide lacks disulfide linkages with Tyr substituted for Cys at residues 8, 11, 34 and 40 and also Leu replacing Met at residues 21 and 28 (Fig. 1). Plot of % conformations assessed from FTIR spectra (black bars) of the B-YL mimic in DPPC:POPC: POPG (mole:mole:mole, 5:3:2) bilayers (Table 1) vs. the corresponding structures calculated from DSSP analysis (i.e., H-bond estimation algorithm) of an MD simulation of B-YL (gray bars) in an aqueous DPPC:POPC:POPG lipid box for 500 nsec. The respective proportions of secondary structures for B-YL obtained from FTIR deconvolution are in good agreement with those determined using MD simulations, with each technique showing α-helix > Loop-Turn ~ Disordered ≥ β-sheet. The absence of any β-sheet in our MD model is shown by the asterisk (*)
