Abstract
Two new phthalide derivatives, rhytidhylides A (1) and B (2), together with ten known compounds (3–12) were isolated from cultures of Rhytidhysteron sp. BZM-9, an endophyte isolated from the leaves of Leptospermum brachyandrum. Their structures were identified by an extensive analysis of NMR, HRESIMS, ECD, and through comparison with data reported in the literature. In addition, the cytotoxic activities against two human hepatoma cell lines (HepG2 and SMMC7721) and antibacterial activities against MRSA and E. coli were evaluated.
Keywords: Leptospermum brachyandrum, Rhytidhysteron sp. BZM-9, endophyte, phthalate derivative
1. Introduction
Endophytic fungi plays a role not only in supplying plants with the basic nutrients indispensable for their growth and helping them in the mechanisms of adaptation to various environmental stresses (i.e., salinity, drought), but they can also produce various bioactive natural products [1]. Phthalide, widely found in several higher and lower plant and fungal genera, is a naturally occurring benzobutyrolactones [2]. Many of the naturally occurring phthalides display different biological activities including antibacterial [3], antifungal [4], insecticidal [5], cytotoxic [6], and antioxidant [7] effects, which has also attracted widespread attention from other researchers.
During our search for new bioactive, secondary metabolites of fungi isolated from various medicinal plants, the strain Rhytidhysteron sp. BZM-9 was obtained and identified, which was isolated from Leptospermum brachyandrum. Rhytidhysteron sp. is a clinically pathogenic fungus [8]. The genus Rhytidhysteron includes two species: R. rufulum and R. hysterinum, which has a worldwide distribution and occurs particularly in the tropics and subtropics [8]. As far as we know, only a few chromones and Spirobisnaphthalenes have been reported from the genus Rhytidhysteron [9,10,11,12]. In our previous work, some chlorinated cyclopentene and isocoumarin derivatives from this strain were reported [13,14]. In the course of our continued search for bioactive compounds from endophytes, two new phthalide derivatives, rhytidhylides A (1) and B (2), along with ten known compounds (3–12), were isolated from cultures of Rhytidhysteron sp. BZM-9 (Figure 1). Herein, we describe the isolation, structure elucidation, and biological activities of these compounds.
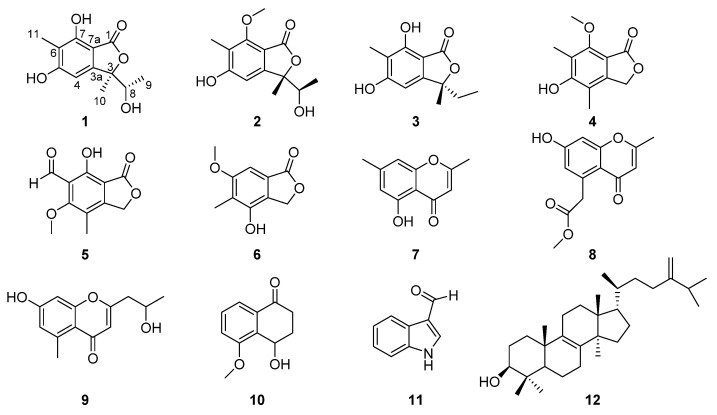
Figure 1.
Structures of compounds 1–12.
2. Results and Discussion
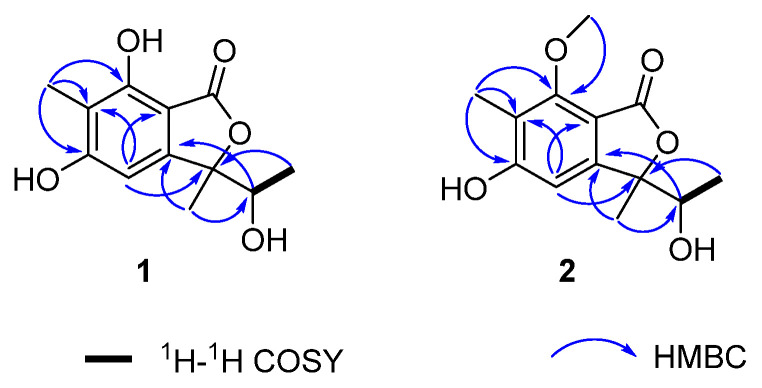
Compound 1 was obtained as a yellow solid, and the molecular formula was deduced as C12H14O5 based on HRESIMS ion peak at m/z 239.0913 [M + H] + (calcd. for C12H15O5, 239.0914 [M + H] +), indicating six degrees of unsaturation. The 1H and 13C NMR data (Table 1) of 1 revealed the presence of one ester carbonyl [(δC 171.6 (C-1)], one five substituted benzene ring [δH 6.41 (H-4); δC 99.9 (C-4), 163.3 (C-5), 111.2 (C-6), 155.4 (C-7), 151.6 (C-3a), 102.8 (C-7a)], one oxymethine [δH 3.87 (q, J = 6.5 Hz, H-8); δC 71.1 (C-8)], one oxygenated sp3 non-protonated carbon [(δC 89.5 (C-3)], and three methyls [δH 2.06 (H-11), 1.57 (H-10), and 1.08 (d, J = 6.5 Hz, H-9); δC 6.4 (C-11), 20.7 (C-10), 16.1 (C-9)]. Comparison of the 1H and 13C NMR data (Table 1) of 1 with those of known compound 3 suggested that both compounds shared similar structural features, with the difference being that one proton of C-8 was replaced by a hydroxyl group. The HSQC correlation of C-8 with a methine proton (δH 3.87) rather than a methylene proton and obvious down-field chemical shift of C-8 (δC 71.1) verified this conclusion. Thus, the planar structure of 1 was completely established, which was further supported by HMBC and 1H-1H COSY correlations, as present in Figure 2.
Table 1.
1H (500 MHz) and 13C NMR (125 MHz) spectral data of 1 and 2 (δ in ppm, J in Hz).
| Position | 1 (CD3OD) | 2 (DMSO-d6) | ||
|---|---|---|---|---|
| δ H | δ C | δ H | δ C | |
| 1 | 171.6 | 167.6 | ||
| 3 | 89.5 | 87.2 | ||
| 4 | 6.41 (1H, s) | 99.9 | 6.69 (1H, s) | 104.5 |
| 5 | 163.3 | 164.1 | ||
| 6 | 111.2 | 118.0 | ||
| 7 | 155.4 | 157.5 | ||
| 8 | 3.87 (1H, q, 6.5) | 71.1 | 3.84 (1H, q, 6.0) | 70.5 |
| 9 | 1.08 (3H, d, 6.5) | 16.1 | 0.90 (3H, d, 6.0) | 17.9 |
| 10 | 1.57 (3H, s) | 20.7 | 1.47 (3H, s) | 23.6 |
| 11 | 2.06 (3H, s) | 6.4 | 2.00 (3H, s) | 9.0 |
| 3a | 151.6 | 153.6 | ||
| 7a | 102.8 | 107.8 | ||
| -OCH3 | 3.86 (3H, s) | 61.7 | ||
Figure 2.
1H-1H COSY and key HMBC correlations of 1 and 2.
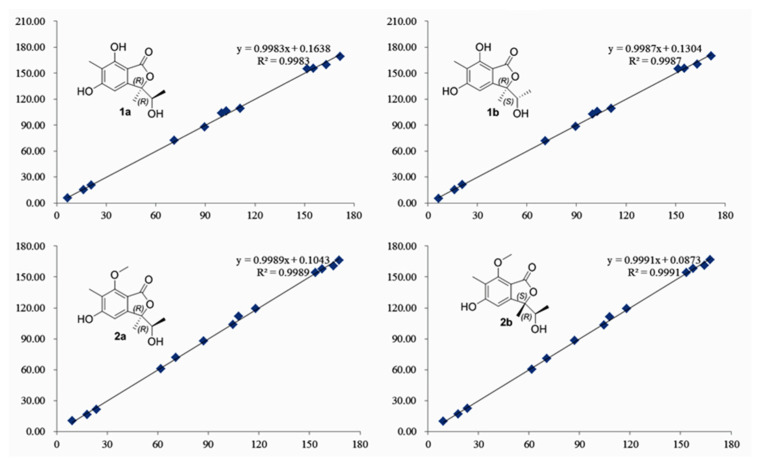
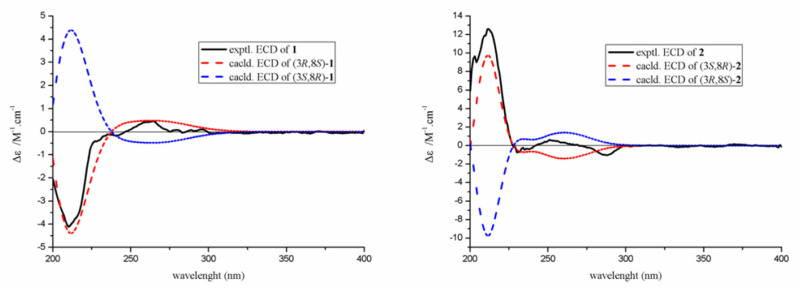
Compound 1 has two asymmetric centers. The NOESY correlations were not conclusive in the case of 1. Thus, to determine the relative configuration of 1, gauge-independent atomic orbital (GIAO) DFT 13C NMR calculations were performed at the ɷB97x-D/6-31G* level using MeOH as the solvent, and the calculations data were compared with their experimental values, following the reported STS protocol. According to linear regression analysis of 13C NMR chemical shifts, the values of the correlation coefficient (R2) were 0.9983 for 1a and 0.9987 for 1b (Figure 3. Moreover, the resulting Pmean and Prel parameters as well as MAE and RMS values further showed 1b or its enantiomers are correct structures for 1 (Table 2). Subsequently, the absolute configurations of 1 were determined to be 3R,8S on the basis that the experimental ECD perfectly matched with the calculated ECD (Figure 4). Therefore, compound 1 was named rhytidhylide A.
Figure 3.
Regression analysis of experimental and calculated 13C NMR chemical shifts for 1 and 2.
Table 2.
Calculated 13C chemical shifts fitting with the experimental data of compounds 1 and 2 following STS protocol.
| Exptl. | 1 | Exptl. | 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1a | dev | 1b | dev | 2a | dev | 2b | dev | ||
| 171.5 | 169.08 | 2.42 | 169.41 | 2.09 | 167.6 | 166.27 | 1.33 | 166.58 | 1.02 |
| 89.3 | 87.98 | 1.32 | 88.47 | 0.83 | 87.2 | 87.86 | 0.66 | 88.21 | 1.01 |
| 100.4 | 103.67 | 3.27 | 102.82 | 2.42 | 104.5 | 103.97 | 0.53 | 103.25 | 1.25 |
| 163.3 | 159.66 | 3.64 | 159.94 | 3.36 | 164.1 | 160.75 | 3.35 | 161.08 | 3.02 |
| 111.1 | 108.95 | 2.15 | 108.95 | 2.15 | 118 | 119.44 | 1.44 | 119.51 | 1.51 |
| 155.1 | 155.49 | 0.39 | 155.69 | 0.59 | 157.5 | 157.92 | 0.42 | 158.15 | 0.65 |
| 70.9 | 72.32 | 1.42 | 71.78 | 0.88 | 70.5 | 71.59 | 1.09 | 71.06 | 0.56 |
| 16.1 | 15.42 | 0.68 | 15.64 | 0.46 | 17.9 | 16.63 | 1.27 | 16.75 | 1.15 |
| 21.6 | 20.84 | 0.76 | 21.63 | 0.03 | 23.6 | 21.54 | 2.06 | 22.41 | 1.19 |
| 6.4 | 5.80 | 0.60 | 5.39 | 1.01 | 9 | 10.31 | 1.31 | 9.99 | 0.99 |
| 151.2 | 154.90 | 3.70 | 154.78 | 3.58 | 153.6 | 154.37 | 0.77 | 154.10 | 0.50 |
| 103.2 | 105.98 | 2.78 | 105.61 | 2.41 | 107.8 | 111.73 | 3.93 | 111.45 | 3.65 |
| 61.7 | 60.63 | 1.07 | 60.46 | 1.24 | |||||
| MAE a | 1.93 | MAE a | 1.65 | MAE a | 1.48 | MAE a | 1.37 | ||
| RMS b | 2.26 | RMS b | 2.00 | RMS b | 1.79 | RMS b | 1.63 | ||
| Pmean | 19.63% | Pmean | 27.76% | Pmean | 26.40% | Pmean | 32.40% | ||
| Prel | 1.54% | Prel | 98.46% | Prel | 6.51% | Prel | 93.49% | ||
a mean absolute error; b root mean square.
Figure 4.
Experimental and calculated ECD spectra of compounds 1 and 2.
Compound 2 was obtained as a pale yellow solid with the molecular formula of C13H16O5, which was deduced from the positive HRESIMS ion as m/z 253.1073 [M + H] + (calcd for C13H17O5, 253.1076 [M + H] +), implying six degrees of unsaturation. Comparison of the 1D and 2D NMR data (Table 1) of 2 and 1 suggested that they shared closely similar NMR resonances. The main difference between them was the exhibition of a methoxy group at the C-7 position of 1 and a hydroxyl group C-7 position of 1. This conclusion could be further verified by the HMBC correlation from -OCH3 (δH 3.86) to C-7 (δC 157.5) (Figure 2). Hence, the planar structure of 2 was corroborated.
Similarly, the relative configuration of 2 was assigned by (GIAO) DFT 13C NMR calculations. By comparing the calculated 13C NMR data with the corresponding experimental values, the possible configuration of 2 was distinguished (Figure 3) (Table 2). Finally, the absolute configuration was established as 3S, 8R based on the experimental ECD, which was highly similar to the calculated ECD (Figure 4). Thus, compound 2 was named rhytidhylide B.
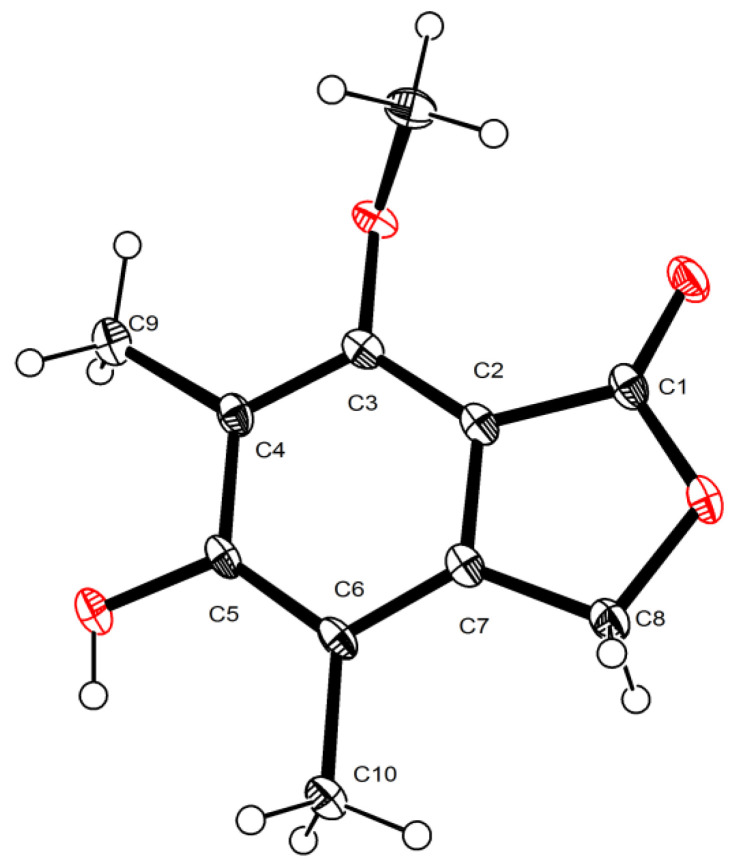
In addition to the two new phthalide derivatives, rhytidhylides A and B, ten known compounds were isolated and identified as 3-ethyl-5,7-dihydroxy-3,6-dimethylphthalide (3) [15], 5-hydroxy-7-methoxy-4,6-dimethylphthalide (4) [16], 4-hydroxy-6-methoxy-7-methyl-3-oxo-1,3-dihy-dro-isobenzofuran-5-carbaldehyde (5) [17], 4-hydroxy-6-methoxy-5-methyl-1(3H)-isobenzofuranone (6) [18], altechromones A (7) [19], 2-methyl-5-methylcarboxymethyl-7-hydroxychromone (8) [20], 2-(2’-hydroxypropyl)-5-methyl-7-hydroxychromone (9) [21], 4-O-methylsclerone (10) [22], 1H-indole-3-carboxaldehyde (11) [23], and euphorbol (12) [24], by comparing their experimental spectral data with the reported spectral data in the literature. In addition, the crystallographic data of compound 4 were reported for the first time (Figure 5).
Figure 5.
ORTEP drawing of the X-ray structures of 4.
A review has reported that phthalide derivatives are present in nature with an enormous spectrum of bioactivities extending from bactericidal to cytotoxic [2]. Additionally, methicillin-resistant Staphylococcus aureus (MRSA) is endemic in hospitals worldwide and causes substantial morbidity and mortality, which is becoming an important public health problem [25]. Therefore, all compounds were evaluated for their cytotoxic activities inhibited by two human hepatoma cell lines (HepG2 and SMMC7721) and antibacterial activities against MRSA and E. coli. Among them, compound 12 displayed weak antibacterial activity against MRSA with a MIC value of 62.5 ug/mL (Table 3) (Figure S15, Supplementary Materials), while none of the compounds showed cytotoxicities at the tested condition (IC50 > 80 µM) (Figure S16, Supplementary Materials).
Table 3.
Antimicrobial activities of compounds 1–12.
| Compounds | MIC (ug/mL) | |
|---|---|---|
| MRSA | E. coli | |
| 1–2, 5, 7 | >500 | >500 |
| 3 | 250 | >500 |
| 4 | 250 | >500 |
| 6 | 125 | >500 |
| 8 | 125 | >500 |
| 9 | 125 | >500 |
| 10 | 125 | >500 |
| 11 | 500 | >500 |
| 12 | 62.5 | >500 |
| Vancomycin a | 1.25 | ≥40 |
a positive control.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were measured on a Rudolph Research Analytical Autopol IV automatic polarimeter. HRESIMS spectra were recorded on an Agilent 6500 series Q-TOF mass spectrometer (Agilent, Singapore) analyser with a positive ion mode. Experimental ECD spectra were performed on a chirascan plus Circular Dichroism spectrometer. NMR spectra (1D and 2D) were obtained using Bruker 500MHz spectrometers and using TMS as an internal standard. The single crystal data were collected on an Agilent Xcalibur Novasingle-crystal diffractometer equipped with CuKα radiation. Preparative HPLC was done using an Agilent 1100 prep-HPLC system with a YMC-peak ODS-A column (5 µm, 250 × 10 mm). Sephadex LH-20 (GE Healthcare, Uppsala, Sweden), silica gel (200–300 and 60–100 mesh, Qingdao Marine Chemical Factory, Qingdao, China), and C18 reversed-phase silica gel (40–75 µm, Fuji, Kasugai, Japan) were used for column chromatography (CC). All solvents were of analytical grade.
3.2. Fungal Material
The fungal strain Rhytidhysteron sp. BZM-9 was isolated from the leaves of Leptospermum brachyandrum, collected in the South China Botanical Garden in Guangzhou city, Guangdong province of China, in September 2016. The strain was deposited at the Xiangya School of Pharmaceutical Sciences, Central South University. The fungus was identified as Rhytidhysteron sp. BZM-9 (GenBank accession number: MN788611).
3.3. Fermentation, Extraction and Isolation of Compounds
The fungus was inoculated on solid rice medium, which was prepared by autoclaving 250 g of rice in 300 mL of demineralized water in a 500 mL Erlenmeyer flask. The fermentation was performed in 15 flasks under static conditions at room temperature for a month [26]. The fungal culture was extracted with ethyl acetate added to each flask, and the extract was subsequently dried under a vacuum to afford 50 g.
The crude extract was subjected to silica gel column chromatography eluting with petroleum ether (PE)–ethyl acetate (EtOAc)–methanol (MeOH) (v/v/v, 100:0:0–0:0:100) to give ten fractions (Fr. 1 to Fr. 10). Fr. 6 (8.2 g) was further purified by silica gel CC with a gradient of CH2Cl2-MeOH (v/v, 100:0~0:100) to provide eight fractions (Fr. 6-1 to Fr. 6-8). Fr. 6-3 (0.83 g) was further purified by semi-preparative HPLC with MeCN-H2O (0~20 min, 25~25%, 3 mL/min) to obtain compound 7 (10.3 mg, tR = 17 min), 8 (3.5 mg, tR = 14 min), and 9 (5.8 mg, tR = 11 min). Fr. 4 was separated by column chromatography over silica gel eluted with PE-EtOAc (v/v, 30:1–0:100) and then purified by prep-HPLC with MeCN-H2O (0~30 min, 80~95%, 3 mL/min) to give 12 (3.4 mg, tR = 16 min).
Fr. 6-4 (1.2 g) was performed on Sephadex LH-20 column with MeOH to get four subfractions (Fr. 6-4-1 to Fr. 6-4-4). Fr. 6-4-1 (0.19 mg) was then purified by semi-preparative HPLC with ACN/H2O (0~50 min, 10~30%) to obtain compounds 1 (3.5 mg, tR = 26.3 min) and 2 (2.3 mg, tR = 29.5 min). Fr. 6-4-2 (0.53 g) was purified by silica gel CC and eluted with a gradient of PE-EtOAc (v/v, 30:1~0:100) to provide four fractions (Fr. 6-4-2-1 to Fr. 6-4-2-4). Fr. 6-4-2-2 (95 mg) was further purified by semi-preparative HPLC using MeCN-H2O (0~40 min, 30~40%, 3 mL/min) to obtain compounds 3 (7.3 mg, tR = 26 min), 4 (2 mg, tR = 13.5 min), and 5 (6.6 mg, tR = 18.4 min). Fr. 6-4-2-2 (0.31 g) was purified by silica gel column chromatography using a mixture system of PE-EtOAc (v/v, 20:1–0:100) to obtain compound 6 (1.6 mg).
Fr. 6-5 (2.3 g) was subjected to a reversed-phase ODS column with a gradient of MeOH-H2O (v/v, 20:80~100:0) to obtain eight subfractions (Fr. 5-1 to Fr. 5-8). Further purification of fraction Fr. 5-2 (0.21 g) by semi-preparative HPLC (mobile phase: 18% MeCN/H2O) yielded compound 11 (2.1 mg, tR = 30 min). Fr. 5-3 (0.95 g) was fractionated into three subfractions (Fr. 5-3-1 to Fr. 5-3-3) by silica gel chromatography (CH2Cl2-MeOH, v/v, 100:0~0:100). Fr. 5-3-1 was further purified by semi-preparative HPLC using MeCN-H2O (0~30 min, 20~30%, 3 mL/min) to obtain compound 10 (5.4 mg, tR = 15 min).
Rhytidhylide A (1). Yellow solid; −11.6 (c 0.18, methanol); HPLC-UV (ACN-H2O) λmax: 226, 262, 295 nm; HRESIMS m/z 239.0913 [M + H] + (calcd for C12H15O5, 239.0914 [M + H] +); 1H (500 MHz) and 13C (125 MHz) NMR spectral data, see Table 1.
Rhytidhylide B (2). Pale yellow solid; +5.0 (c 0.10, methanol); HPLC-UV (ACN-H2O) λmax: 220, 262, 295 nm; HRESIMS m/z 253.1073 [M + H] + (calcd for C13H17O5, 253.1076 [M + H] +); 1H (500 MHz) and 13C (125 MHz) NMR spectral data, see Table 1.
3.4. X-ray Crystallographic Analysis
Crystal data for 4. (No. CCDC 2104595) C11H12O4 (M = 208.21 g/mol, orthorhombic, space group Pna21 (no. 33), a = 7.6421(3) Å, b = 16.5289(5) Å, c = 7.7031(2) Å, V = 973.02(5) Å3, Z = 4, T = 99.9(6) K, μ(CuKα) = 0.910 mm−1, Dcalc = 1.421 g/cm3, 3840 reflections measured (10.704° ≤ 2Θ ≤ 147.408°), 1671 unique (Rint = 0.0541, Rsigma = 0.0359) which were used in all calculations. The final R1 was 0.0566 (I > 2σ(I)) and wR2 was 0.1561 (all data). Flack parameter = −0.2(3).
3.5. Quantum Chemistry Calculations
The conformation optimization, ECD spectrum calculation, and DFT GIAO 13C NMR calculation were performed as previously described [14].
3.6. Cytotoxic Activity Assay
Cytotoxicities of all compounds were tested against two human hepatoma cell lines (HepG2 and SMMC7721) using a microplate 3-(4,5-dimethylth-iazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously [13]. Doxorubicin was used as a positive control, and experiments were repeated three times.
3.7. Antimicrobial Activity Assay
Antimicrobial activities of all compounds were evaluated by calculating MIC values against MRSA and E. coli using the broth microdilution method according to CLSI guidelines [27]. Vancomycin (MIC = 1.25 µg/mL) was used as a positive control.
4. Conclusions
In the course of our continued exploration of the fungal strain Rhytidhysteron sp. BZM-9 for biologically active metabolites, two new phthalide derivatives, rhytidhylides A (1) and B (2), together with ten known compounds (3-), were isolated and identified. The cytotoxic activities and antimicrobial activities of 1–12 were also evaluated, but only compound 12 showed weak inhibitory activity against MRSA. All in all, the activities of these compounds were not thoroughly investigated, just the evaluation of the cytotoxicity and antibacterial assays, and only a few cells and strains have been tested. Other activities and their mechanisms are still worthy of further exploration.
Supplementary Materials
The following are available online, Figure S1: HRESIMS spectrum of compound 1; Figure S2: 1H NMR spectrum of compound 1; Figure S3: 13C NMR spectrum of compound 1; Figure S4: 1H-1H COSY spectrum of compound 1; Figure S5: HSQC spectrum of compound 1; Figure S6: HMBC spectrum of compound 1; Figure S7: NOESY spectrum of compound 1; Figure S8: HRESIMS spectrum of compound 2; Figure S9: 1H NMR spectrum of compound 2; Figure S10: 13C NMR spectrum of compound 2; Figure S11: 1H-1H COSY spectrum of compound 2; Figure S12: HSQC spectrum of compound 2; Figure S13: HMBC spectrum of compound 2; Figure S14: NOESY spectrum of compound 2; Figure S15: Antimicrobial activity assay results; Figure S16: Dose-response curves for human hepatoma cell lines (HepG2 and SMMC7721).
Author Contributions
Conceptualization, Z.Z.; methodology, S.Z., D.C. and M.K.; software, S.Z. and D.C.; validation, Z.Z., K.X. and S.Z.; formal analysis, W.P. and F.K.; investigation, Y.C.; resources, J.T.; data curation, S.Z., D.C. and Z.Z.; writing—original draft preparation, S.Z., D.C. and Z.Z.; writing—review and editing, S.Z., D.C. and Z.Z.; visualization, S.Z.; supervision, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Hunan Province (No. 2021JJ30917), the High-tech Industry Science and Technology Innovation Project of Hunan Province (2020GK4083), the Hunan Province Ordinary Higher Education Teaching Reform Research Project, the Postgraduates Innovation Program of Central South University (No. 1053320212747; 1053320212725), the Central South University postgraduate independent exploration and innovation project (No. 2021zzts0978; 2021zzts0994), and the Open Sharing Fund for the Large-scale Instruments and Equipments of Central South University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available in this publication and in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kuzniar A., Wlodarczyk K., Wolinska A. Agricultural and other biotechnological applications resulting from trophic plant-endophyte interactions. Agronomy. 2019;9:779. doi: 10.3390/agronomy9120779. [DOI] [Google Scholar]
- 2.Leon A., Del-Angel M., Avila J.L., Delgado G. Phthalides: Distribution in nature, chemical reactivity, synthesis, and biological activity. Prog. Chem. Org. Nat. Prod. 2017;104:127–246. doi: 10.1007/978-3-319-45618-8_2. [DOI] [PubMed] [Google Scholar]
- 3.Brady S.F., Wagenaar M.M., Singh M.P., Janso J.E., Clardy J. The cytosporones, new octaketide antibiotics isolated from an endophytic fungus. Org. Lett. 2000;2:4043–4046. doi: 10.1021/ol006680s. [DOI] [PubMed] [Google Scholar]
- 4.Katoh N., Nakahata T., Kuwahara S. Synthesis of novel antifungal phthalides produced by a wheat rhizosphere fungus. Tetrahedron. 2008;64:9073–9077. doi: 10.1016/j.tet.2008.07.023. [DOI] [Google Scholar]
- 5.Miyazawa M., Tsukamoto T., Anzai J., Ishikawa Y. Insecticidal effect of phthalides and furanocoumarins from Angelica acutiloba against Drosophila melanogaster. J. Agric. Food Chem. 2004;52:4401–4405. doi: 10.1021/jf0497049. [DOI] [PubMed] [Google Scholar]
- 6.Mullady E.L., Millett W.P., Yoo H.D., Weiskopf A.S., Chen J., DiTullio D., Knight-Connoni V., Hughes D.E., Pierceall W.E. A phthalide with in vitro growth inhibitory activity from an oidiodendron strain. J. Nat. Prod. 2004;67:2086–2089. doi: 10.1021/np040123n. [DOI] [PubMed] [Google Scholar]
- 7.Tianpanich K., Prachya S., Wiyakrutta S., Mahidol C., Ruchirawat S., Kittakoop P. Radical scavenging and antioxidant activities of isocoumarins and a phthalide from the endophytic fungus Colletotrichum sp. J. Nat. Prod. 2011;74:79–81. doi: 10.1021/np1003752. [DOI] [PubMed] [Google Scholar]
- 8.Mahajan V.K., Sharma V., Prabha N., Thakur K., Sharma N.L., Rudramurthy S.M., Chauhan P.S., Mehta K.S., Abhinav C. A rare case of subcutaneous phaeohyphomycosis caused by a Rhytidhysteron species: A clinico-therapeutic experience. Int. J. Dermatol. 2014;53:1485–1489. doi: 10.1111/ijd.12529. [DOI] [PubMed] [Google Scholar]
- 9.Pudhom K., Teerawatananond T., Chookpaiboon S. Spirobisnaphthalenes from the mangrove-derived fungus Rhytidhysteron sp. AS21B. Mar. Drugs. 2014;12:1271–1280. doi: 10.3390/md12031271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pudhom K., Teerawatananond T. Rhytidenones A-F, Spirobisnaphthalenes from Rhytidhysteron sp. AS21B, an endophytic fungus. J. Nat. Prod. 2014;77:1962–1966. doi: 10.1021/np500068y. [DOI] [PubMed] [Google Scholar]
- 11.Chokpaiboon S., Choodej S., Boonyuen N., Teerawatananond T., Pudhom K. Highly oxygenated chromones from mangrove-derived endophytic fungus Rhytidhysteron rufulum. Phytochemistry. 2016;122:172–177. doi: 10.1016/j.phytochem.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Siridechakorn I., Yue Z.W., Mittraphab Y., Lei X.G., Pudhom K. Identification of spirobisnaphthalene derivatives with anti-tumor activities from the endophytic fungus Rhytidhysteron rufulum AS21B. Bioorgan. Med. Chem. 2017;25:2878–2882. doi: 10.1016/j.bmc.2017.02.054. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S., Kang F.H., Tan J.B., Chen D.K., Kuang M., Wang W.X., Xu K.P., Zou Z.X. (±)-Rhytidhymarins A and B, two pairs of new isocoumarin derivatives from endophytic fungus Rhytidhysteron sp. BZM-9. New J. Chem. 2021;45:12700–12704. doi: 10.1039/D1NJ01993G. [DOI] [Google Scholar]
- 14.Zhang S., Wang W.X., Tan J.B., Kang F.H., Chen D.K., Xu K.P., Zou Z.X. Rhytidhyesters A-D, four new chlorinated cyclopentene derivatives from the endophytic fungus Rhytidhysteron sp. BZM-9. Planta Med. 2021;87:489–497. doi: 10.1055/a-1429-3396. [DOI] [PubMed] [Google Scholar]
- 15.Klaiklay S., Rukachaisirikul V., Aungphao W., Phongpaichit S., Sakayaroj J. Depsidone and phthalide derivatives from the soil-derived fungus Aspergillus unguis PSU-RSPG199. Tetrahedron Lett. 2016;57:4348–4351. doi: 10.1016/j.tetlet.2016.08.040. [DOI] [Google Scholar]
- 16.Sommart U., Rukachaisirikul V., Tadpetch K., Sukpondma Y., Phongpaichit S., Hutadilok-Towatana N., Sakayaroj J. Modiolin and phthalide derivatives from the endophytic fungus Microsphaeropsis arundinis PSU-G18. Tetrahedron. 2012;68:10005–10010. doi: 10.1016/j.tet.2012.09.043. [DOI] [Google Scholar]
- 17.Tang Y.J., Wang Y.M., Wang Z.H., Zhang J., Qin J.C., Wang Q.K., Yang L.H., Xu L.Z., Ding Y.L., Guo Y., et al. Novel antimicrobial metabolites produced by Sika deer-associated Actinomyces sp. JN411010. Nat. Prod. Res. 2013;27:2183–2189. doi: 10.1080/14786419.2013.811658. [DOI] [PubMed] [Google Scholar]
- 18.Martin J.A., Vogel E. The synthesis of zinniol. Tetrahedron. 1980;36:791–794. doi: 10.1016/S0040-4020(01)93696-8. [DOI] [Google Scholar]
- 19.Kimura Y., Mizuno T., Nakajima H., Hamasaki T. Altechromones A and B, new plant growth regulators produced by the fungus, Alternaria sp. Biosci. Biotech. Biochem. 1992;56:1664–1665. doi: 10.1271/bbb.56.1664. [DOI] [Google Scholar]
- 20.Xu W.F., Chen G., Li Z.Q., Lu X., Pei Y.H. Isolation and identification of chemical constituents from Rheum palmatum L. Shenyang Yaoke Daxue Xuebao. 2013;30:837–839. [Google Scholar]
- 21.Khamthong N., Rukachaisirikul V., Tadpetch K., Kaewpet M., Phongpaichit S., Preedanon S., Sakayaroj J. Tetrahydroanthraquinone and xanthone derivatives from the marine-derived fungus Trichoderma aureoviride PSU-F95. Arch. Pharm. Res. 2012;35:461–468. doi: 10.1007/s12272-012-0309-2. [DOI] [PubMed] [Google Scholar]
- 22.Chang C.W., Chang H.S., Cheng M.J., Liu T.W., Hsieh S.Y., Yuan G.F., Chen I.S. Inhibitory effects of constituents of an endophytic fungus Hypoxylon investiens on nitric oxide and interleukin-6 production in RAW264.7 macrophages. Chem. Biodivers. 2014;11:949–961. doi: 10.1002/cbdv.201300364. [DOI] [PubMed] [Google Scholar]
- 23.Hassan W., Edrada R., Ebel R., Wray V., Proksch P. New alkaloids from the mediterranean sponge Hamigera hamigera Mar. Drugs. 2004;2:88–100. [Google Scholar]
- 24.Li F., Li K., Li X.M., Wang B.G. Chemical constituents of marine algal-derived endophytic fungus Exophiala oligosperma EN-21. Chin. J. Oceanol. Limnol. 2011;29:63–67. doi: 10.1007/s00343-011-9949-1. [DOI] [Google Scholar]
- 25.DeLeo F.R., Otto M., Kreiswirth B.N., Chambers H.F. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–1568. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu H.M., Jiang X.L. Structure and properties of bacterial cellulose produced using a trickling bed reactor. Appl. Biochem. Biotechnol. 2014;172:3844–3861. doi: 10.1007/s12010-014-0795-4. [DOI] [PubMed] [Google Scholar]
- 27.Li C.R., Zhai Q.Q., Wang X.K., Hu X.X., Li G.Q., Zhang W.X., Pang J., Lu X., Yuan H., Gordeev M.F., et al. In vivo antibacterial activity of MRX-I, a new oxazolidinone. Antimicrob. Agents Chemother. 2014;58:2418–2421. doi: 10.1128/AAC.01526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available in this publication and in the Supplementary Materials.