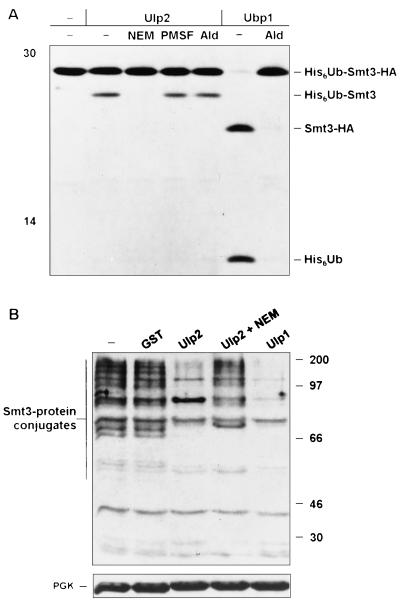
FIG. 2.
S. cerevisiae Ulp2 is an Smt3-cleaving enzyme. (A) Cleavage of radiolabeled His6-ubiquitin-Smt3-HA by GST-Ulp2 and yeast Ubp1 analyzed after 2 h at 30°C by SDS-polyacrylamide gel electrophoresis (12.5% gel). Left lane, no added enzyme. Inhibitor preincubations were done for 15 min at room temperature. Ald, ubiquitin aldehyde; −, absent. (B) In vitro cleavage by purified GST-Ulp2 or GST-Ulp1 of yeast Smt3-protein conjugates. The blotting conditions did not allow visualization of free Smt3. NEM-treated extracts (25 μg) from ulp2Δ cells grown at 23°C (two left lanes) or 37°C (three right lanes) are shown. A species of ∼85 kDa was reproducibly enhanced following Ulp2 digestion in vitro; this might represent a multiply modified protein from which not all Smt3 molecules were cleaved. A rabbit anti-Smt3 antibody was used for immunoblot analysis. In the lower gel, filters were reprobed with anti-PGK (3-phosphoglycerate kinase) to compare protein loadings. The positions of molecular mass markers are indicated in each panel (in kilodaltons).