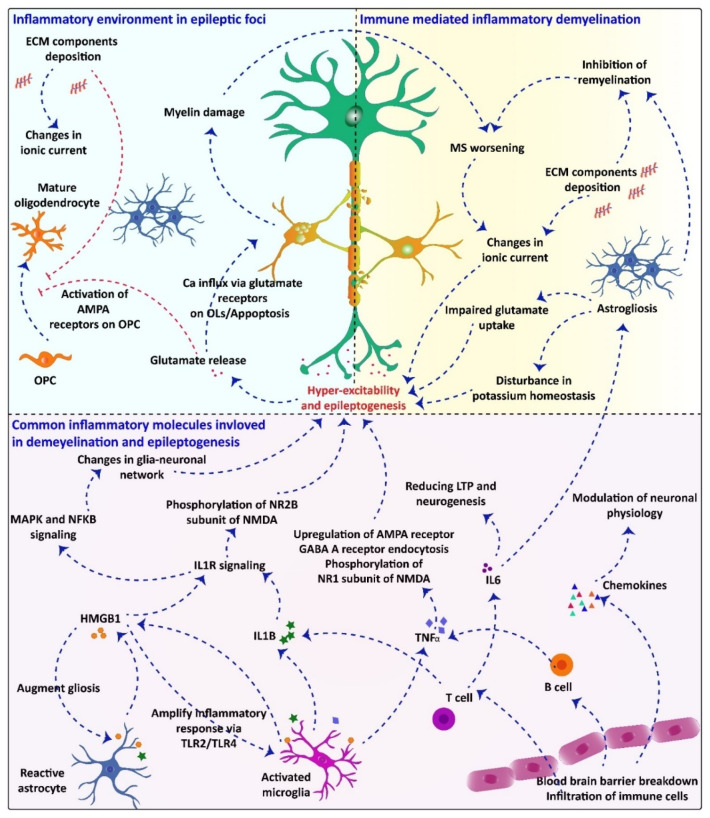
Figure 1.
The common inflammatory mechanism underlying demyelination and epilepsy and how one can pathology accelerate the other is proposed: Inflammatory processes in MS pathology are also involved in the etiology of seizures and trigger hyper-excitability. In turn, inflammation, gliosis, ECM component deposition, blood–brain barrier breakdown, immune cells infiltration, and production of inflammatory mediators such as IL-1B, IL-6, HMGB1, TNF-α, and chemokines might stabilize a common mechanism in the pathophysiology of seizures and epilepsy in MS. Phosphorylation of NR2 subunit of NMDA receptor by IL-1B and HMGB1 may lead to an enhanced NMDA activity and Ca2+ influx into neurons. Different types of cytokines are released by infiltrated or resident immune cells within the demyelinated lesions. These cytokines can modulate neuronal physiology by changing voltage-gated channels and enhancing discharge of some neurotransmitters. Furthermore, pro-inflammatory cytokines and IL-1B inhibit astrocytic glutamate uptake, leading to hyper-excitability and, subsequently, seizures in the context of MS. Releasing high amount of glutamate and impaired glutamate uptake can induce apoptosis in oligodendrocytes via influx of large amount of Ca2+ through glutamate receptors. This process exacerbates myelin damage and worsens MS.