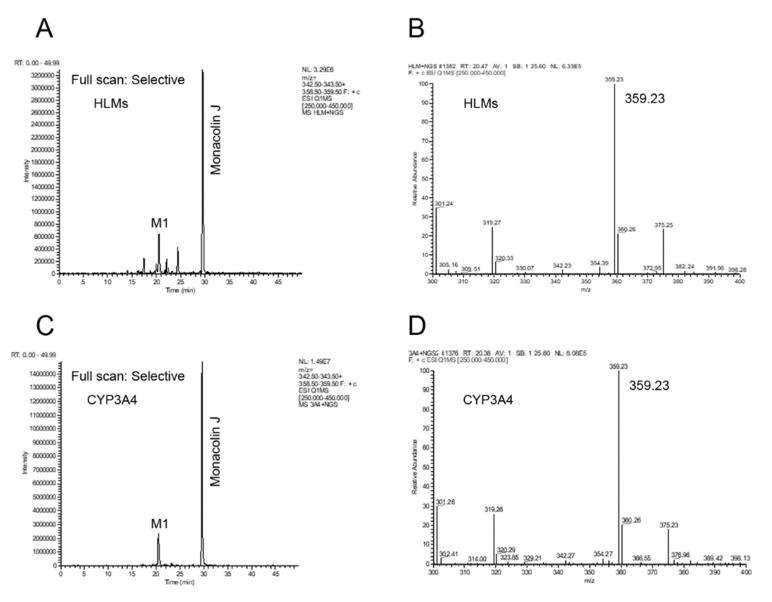
Figure 8.
LC–MS results of the metabolite of monacolin J when using HLMs and CYP3A4. LC–MS chromatogram of monacolin J that was catalyzed using HLMs (A) and CYP3A4 (C) in the presence of NADPH. The MS spectra demonstrated that the m/z value of the protonated molecular ion of the major product from both of HLMs (B) and CYP3A4 (D) was 359, which is the same as that produced with M697.