Figure 1. LLPS regulates cGAS signaling.
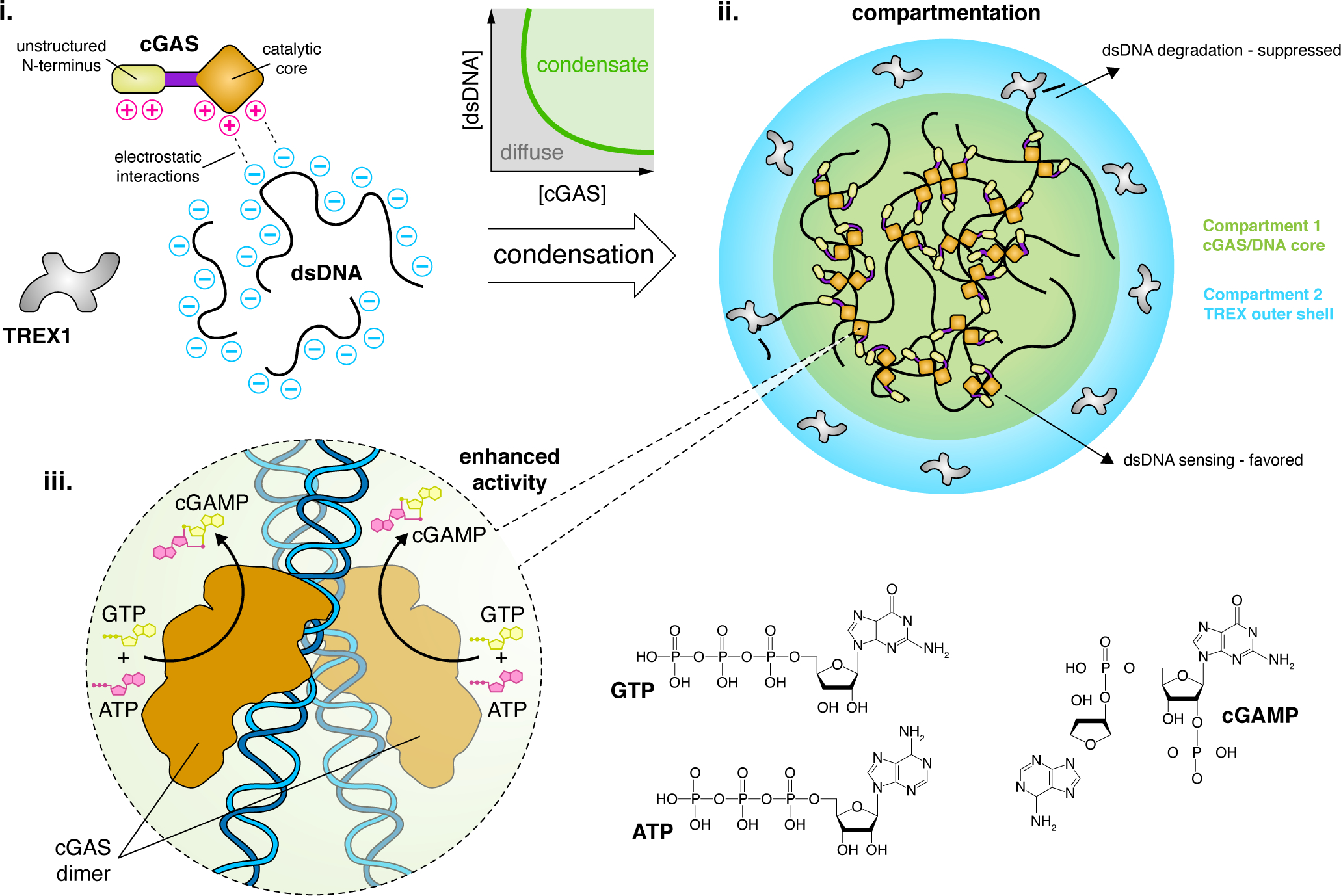
(i) Cytosolic LLPS of cGAS is driven by several factors, including multivalent charge-charge interactions with dsDNA. These properties drive cGAS to undergo an abrupt phase transition to form condensates with dsDNA at a low threshold concentration. (ii) LLPS compartmentalizes the cGAS signaling machinery, forming a core cGAS/DNA condensate that is surrounded by a phase-separated outer shell containing TREX1, a dsDNA-degrading enzyme that antagonizes cGAS signaling. Depletion of dsDNA from the outer shell helps suppress TREX1 function and promote cGAS-mediated DNA sensing. The concentrated environment within the cGAS/DNA core dramatically enhances cGAS activity by increasing DNA-binding efficiency. (iii) cGAS self-associates via its core catalytic domain to form dimers along dsDNA strands, which activates cGAS to catalyze the conversion of GTP and ATP into cGAMP and induce additional downstream signaling.
