Abstract
Microorganisms live in the human digestive system and the gut microbiome constitutes part of our prime determining component for healthy aging and wellness. Gut microbiota has broad influences on its host, beginning from the digestion of food and nutrients absorption to protective roles against invading pathogens and host immune system regulation. Dysbiosis of the gut microbial composition has been linked to numerous diseases and there is a need to have a better grasp on what makes a ‘good’ gut microbiome. Caenorhabditis elegans (C. elegans) model organism is considered as a well-suited in-vivo model system and, is at the frontline of probiotic research because of its well-defined characteristics and prolific nature. Most importantly, C. elegans feeds on bacteria, which speeds up manipulations and investigations in probiotics research tremendously. With its unique salient features of short lifespan, and ease of propagation, different unknown probiotics biological roles can be measured at an organism level with precision in the form of worm’s stress responses, survivability, and lifespan. In this review, new insights on the different mechanisms underlying the establishment of probiotics regulations of conserved signalling pathways such as p38 MAPK/SKN-1, DAF-2/DAF-16, and JNK-1/DAF-16 is highlighted based on information obtained from C. elegans studies. Along with the current state of knowledge and the uniqueness of C. elegans as a model organism, explorations of its future contribution and scope in synthetic biology and probiotics engineering strains are also addressed. This is expected to strengthen our understanding of probiotics roles and to facilitate novel discovery and applications, for specific therapeutics against age-related disorders and various pathophysiological conditions.
Keywords: Caenorhabditis elegans, Probiotics, Signalling, Lactobacilli, Aging
Introduction
Living organisms exist in an intimate relationship that is finely tuned with diverse species, particularly microorganisms. Under the influence of environmental and other intrinsic factors, the host-microorganism relationships can either be advantageous or disadvantageous depending on the fitness, mutualistic association, or dependency of both. Interactions between the host and its microbiome happen primarily along the mucosal surfaces in warm-blooded animals, with the most significant one being the intestinal mucosa. The digestive system contains a vast population of harmonious microorganisms speaking to the host somatic cells' approximate ten folds. Furthermore, approximately 10% of the mammalian bloodstream metabolites were acquired from bacterial sources [1]. A noteworthy aspect of the gut microbiome and human connection is that the observed relationship is dynamic and complex rather than a mere unidirectional of “cause and effect.”
The term “Probiotics” is commonly expounded as “live microbes that, when supplied in appropriate amounts, promote the well-being of the host” (FAO/WHO Probiotics in Food Health and nutritional properties and guidelines for evaluation). Elie Metchnikoff, “Father of natural immunity”, proposed that regular intake of fermented milk (yogurt) that contains probiotics could protect against the harmful effects of pathogenic bacteria and contributes to longevity [2]. The intake of probiotics is linked with the declined survival of notorious organisms such as Helicobacter pylori (H. pylori) in the gastrointestinal tract, and probiotic-mediated therapy for inflammatory bowel disease. [3]. Probiotic bacterial strains are well known to have the capability to synthesize enzymes, peptic toxins like bacteriocins, and signalling molecules like gamma-aminobutyric acid. Numerous findings predicted the functions of probiotics and other plant-based products rich in probiotics for delaying the aging process [4].
Most of the bacterial species recognized with probiotic characteristics present in human beings' digestive tract are linked with the genera Lactobacillus and Bifidobacterium. Similarly, bacteria belonging to genera such as Bacillus, Enterococcus, and yeast Saccharomyces were also conferring health benefits [5]. Probiotic species were found to be competing against other pathogenic microorganisms that are located within the intestine through different mechanisms. These include synthesis of antimicrobial substances and bacteriocins secretion through competition against pathogenic organisms for nutrition and binding surfaces, and their involvement in enriching host mucosal barrier integrity and regulation of the immune system. Many reviews highlighted the constructive impacts of these microbes on both animals and human well-being, incorporating improved processing and supplement intake [4, 6]. Treatment using probiotics symbolize a substitute method for alimentary dysfunction and other diseases that are not connected with the digestive tract. Numerous lactic acid bacteria (LAB) strains were identified and commercialized in probiotics supplementation [7]. It must be noted that the positive effects of probiotics were exclusively strain-dependent, and discrete strains of the same species might exhibit variant impacts. Therefore, utmost care needs to be given while characterizing the fresh probiotics to promote strain specificity. Furthermore, complete insights into the molecular mechanisms using a suitable model organism that can direct varied host-microbiota interplay would contribute necessary measures for using or changing the microbiota and promoting wellness and treatment strategies.
Impact of the Gut Microbiome on Human Health and Diseases
In recent years, with a surge of interests and research activities in intestinal microbiota and human host, a plethora of evidence pointed out that milliard of living microbes present in the digestive tract has significant and unthinkable connections related to human wellness and disease status [8, 9]. Probiotics are known to have modulatory and regulatory roles on every aspect of cell function, from specific genes' transcriptional activity to activation of specific pathways. The connections mentioned above of intestinal microbiota and individual metabolic status deserve particular emphasis. For example, distinct bacteria's roles in regulating the amount of blood sugar and low-grade chronic inflammation in Type 2 Diabetes were reported [10]. Moreover, certain probiotics can help in the surging of IL-10 generation, a pivotal modulatory and anti-inflammatory cytokine in diabetic mice. A rise in IL-10 was depicted to reduce the pro-inflammatory cytokines such as IFN-γ and IL-2/IL-1β, leading to an elimination of low-grade inflammation before the onset of diabetes [11]. Routine intake of probiotic-based yogurt was also reported to potentially reduce the inflammatory markers in pregnant women concerning lowering their CRP level, which is also involved in Type 2 diabetes [12]. Some of the probiotic strains can reduce the oxidative stress in pancreatic cells, thereby reducing the chronic inflammation and programmed cell death in pancreatic cells [13]. Additionally, probiotics were noted to minimize the total and LDL cholesterol level in serum through the regulation of lipids' metabolic reactions, which could potentially be regarded as a determinant for the improvement of Type 2 diabetes [14].
Disease conditions like abnormal cell proliferation, inflammation, excessive body weight and fat deposits over arteries, increased blood pressure, persistent malfunctioning of the heart and kidney were also correlated with the aberrant changes of the gut ecosystem [15, 16]. These findings highlighted that the gut microbiome could maintain an interdependent association towards the host, accomplishing a critical function destined for human wellness. It was also established that a disease condition is often represented by the dysbiosis of the microbial composition along the gastrointestinal tract. Therefore, exploitation of these microbes by probiotics, prebiotics, and synbiotics is a fascinating idea to improve and maintain health. Treatment using probiotics symbolize a substitute method for alimentary dysfunction and other diseases not connected with the digestive tract [17]. Furthermore, complete insights into the molecular mechanisms that directly varied host-microbiota interplay would contribute necessary measures for using or changing the microbiota to promote wellness and treatment strategies.
C. elegans as a Model Organism for Probiotic Studies
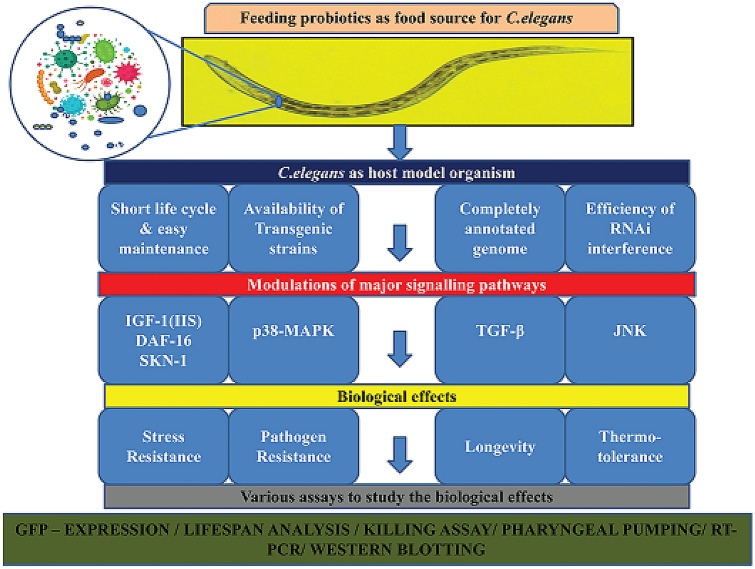
The roundworm Caenorhabditis elegans (C. elegans), which consumes bacteria as a food source, is rapidly becoming popular as an in vivo whole animal model system to understand bacteria-mediated impacts on better bodily functions of the host. It is referred to as an “interspecies model” because the nematode cell lineages, as well as the bacteria it ingests, can be genetically traced [18]. As multicellular organisms, worms have well- defined tissues and anatomy, with its intestine considered to be its most significant body part convenient for host-microbiota studies as it harbors microbes altogether (Fig. 1). Different bacterial feedings have been found to regulate the lifespan of C. elegans by inciting distinctive host responses. While some pathogens are reported to have shortened the lifespan of the worms, many microbes exhibit propitious consequences like a prolongation of life resulting in the anti-aging properties [19, 20]. The nematode’s transparent body allows for easy visualization over its internal organs, fat accumulation, gut colonization of fluorescently tagged bacteria, phenotypic characterization of cells, and other phenotyping changes in pathologies. Apart from this, many other factors attributes to C. elegans as a prolific model system, which includes, the pre-eminent genetic make-up comprising of completely sequenced and annotated genome, ease of assays using available genomic tools to create gene knockout mutants and transgenic strains. The availability of genome-wide screens aided with the efficiency of RNA interference (RNAi), and the exploitability of fluorescently tagged markers enables investigators to monitor gene expression in real-time and in live organisms [21–23]. Most importantly, the nematode's fundamental biological systems, aside from the metabolic reactions, were deeply related to humans with the identification of a high percentile of novel human genes conserved in C. elegans as homologs or orthologs [24]. Numerous studies on probiotics that can produces beneficial effects in mice and humans are found to exert similar effects in C. elegans too [20, 62]. On account of this, C. elegans was esteemed to be an outstanding model organism to uncover bacteria manifesting various probiotic characteristics, health, and anti-aging features (Fig. 2).
Fig. 1.
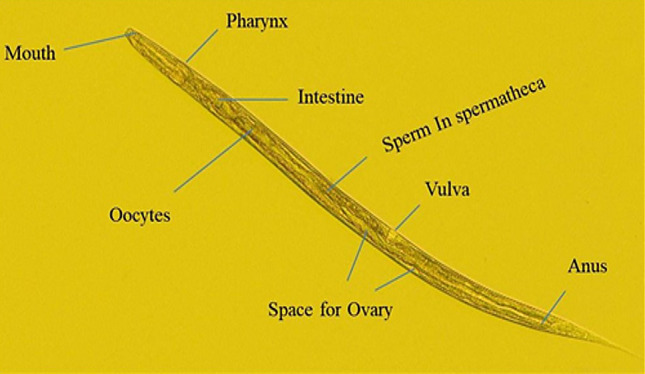
An adult C. elegans hermaphrodite with its key anatomical features
Fig. 2.
Overview of C. elegans as a model organism with simplified representation of approaches employing in probiotics research
The Natural Microbiome and Diet of C. elegans
The main nutriment for C. elegans is bacteria E. coli OP50 under laboratory conditions, although other bacterial strains could be observed in the intestine of the animals existing in the wild. Using high-throughput sequencing for 16S rDNA and sample collection worldwide, researchers have highlighted the varieties of bacterial species that the worms can ingest. Additionally, approach has also been made in systematic sequencing of the intestinal microbes of worms grown in an ambiance created using soil and rotten fruits resembling their natural habitat. The ones isolated from the wild have also revealed the gut microbiome's composition in C. elegans [25]. Although the growing environment plays a more significant role for specificity and in shaping the microbiome of C. elegans, however, are dominated by Proteobacteria, Bacteroidetes, Firmicutes, and Actinobacteria. Plenitude of Enterobacteriaceae, Pseudomonadaceae, Xanthomonadaceae, and Sphingobacteriaceae was also discovered, signifying that the nematode bears a microbial composition that could be embraced with multifarious strains demonstrating extensive diversity [26].
Free-living nematodes could also confront a vast number of unfavourable/detrimental microbial species like Comamonas, Bacillus megaterium, Micrococcus luteus, and various types of Pseudomonas species from time to time [27]. Thus C. elegans serves as a favourable model system to experimentally elucidate the microbiome's role in host evolution and how it adapts to the changing environments like during infection. A trilateral cluster of intertwined tooth-like frameworks is present in the form of an adept grinder at the terminal of its pharyngeal bulb. All the bacterial food sources were processed and passed via the pharynx to the gut. It was observed that most worms would harbor a part of live bacteria by day two of their adulthood. Thus, it renders C. elegans a premier system to examine intestinal microbes. The worm is cultured on Petri plates enclosing a lawn of bacteria of interest that is further scrutinized for gut colonization, development, and other observable changes.
Major C. elegans Signalling Pathways and its Modulation by Probiotics
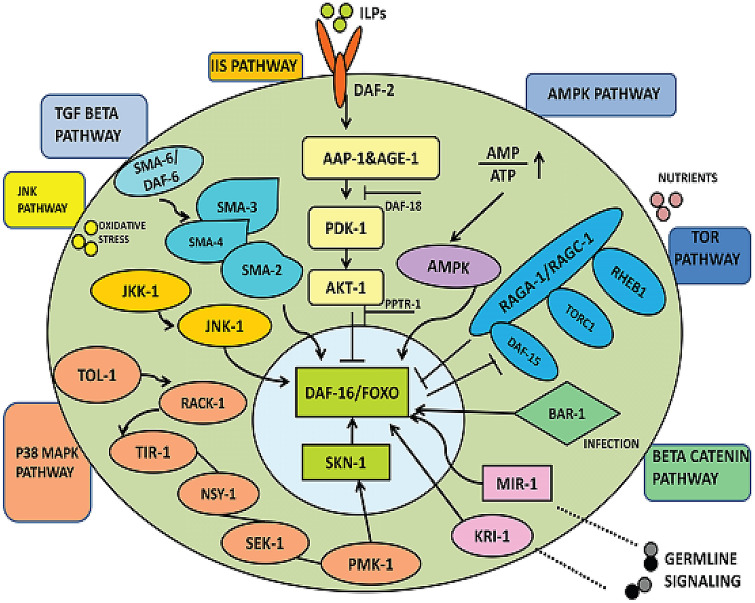
The credibility of C. elegans as a model organism in probiotic research and in elucidating the different molecular mechanisms involved has been linked to its highly conserved signalling pathways and defence systems similar to that of higher mammalian systems. Probiotics influence both the senescence and immune responses of C. elegans under the aegis of evolutionarily conserved pathways. Some of the most commonly reported pathways include the abnormal Dauer Formation/insulin/insulin-like growth factor (DAF/IIS) pathway, p38 mitogen-activated protein kinase (p38 MAPK) pathway, and the transforming growth factor-β (TGF-β) signaling pathway [28, 29]. Indeed, MAPK and IIS pathways act as crucial molecular cascades/switches involving modulating innate immunity, stress responses, and senescence progress in the worms [19] (Fig. 3).
Fig. 3.
Schematic representation of the most common signalling pathways influenced by probiotic strains employed in C. elegans studies. Abbreviations used: (1) IIS (insulin/insulin-like growth factor-1) pathway: DAF-2: dauer formation 2, PDK-1: phosphoinositide-dependent kinase 1, AKT-1: serine/threonine protein kinase DAF-16: dauer formation 16. (2) AMPK: 5′ AMP-activated protein kinase) pathway. (3) β-catenin pathway: BAR-1: β-catenin/armadillo Related-1; DBL-1: DPP/BMP-Like-1. (4) JNK pathway: JKK-1: c-Jun N-terminal kinase kinase; JNK-1: c-Jun N-terminal kinase. (5) p38 MAPK pathway: MAPK: mitogen-activated protein kinase; NSY-1: neuronal symmetry-1; PMK-1: p38 mitogen-activated protein kinase-1; RACK-1: receptor activated protein C kinase; SEK-1: SAPK/ERK kinase-1; SMA: small; TIR-1: toll interleukin-1 receptor-1.5. (6) Germline signalling: MIR1: microRNA1; KIR: Krev interaction trapped/cerebral cavernous malformation 1
Insulin/IGF-1 Signalling (IIS) Pathway
In C. elegans, the IIS pathway is initiated by activating an insulin/insulin-like growth factor-1 receptor ortholog known as dauer formation 2 (DAF-2). This subsequently amplifies phosphorylating events wherein; downstream mediators and specific kinases are activated. Thus, phosphatidylinositol 3-kinase (AGE-1), phosphoinositide-dependent kinase (PDK)-1, and various serine/threonine protein kinases (AKT-1, AKT-2, and SGK-1) are activated. Subsequently, inactivation through phosphorylation of DAF-16, an ortholog of mammalian Forkhead Box O transcription factor (FOXO), takes place with its ultimate sequestration in the cytoplasm through interactions with 14–3-3 proteins [30]. On the contrary, under unfavourable or stressful conditions and/or if there is a defect in any components of the IIS pathway, inactivation or failure of signals to transduce DAF-16 phosphorylation would lead to DAF-16 activation and, its delocalization/enhanced translocation to the nuclei. Once inside the nucleus, DAF-16 can transactivate expressions of series of genes necessary for organism resistance to different kinds of stresses and as required for its survival and longevity [31]. Investigation of the function of DAF-2, DAF-16, and insulin-like signal transduction over Bifidobacterium longum BB68 (BB68), a novel probiotic strain isolated from a centenarian in nematodes, disclosed that DAF-16 commands the transcription of multiple antioxidants and chaperone genes which tend to slow down the progress of aging. The investigators further confirmed this through the reduced longevity observed in worms with mutation of DAF-16 and an enhanced nuclear translocation of DAF-16 compared with those worms supplied with E. coli OP50 only. Additionally, as a downstream target specific to DAF-16, a considerable rise in the expression level of superoxide dismutase 3 (SOD-3) (2.27-fold compared to those supplied with E. coli OP50) was also reported in nematodes supplied with BB68 for 24 h. Therefore, this study highlighted that DAF-16 was involved in the longevity effect of BB68 [32].
DAF 16 as a Transcription Factor
DAF-16 serves as a pivotal central regulator for an organism’s response to different kinds of stresses. To orchestrate these other processes, DAF-16 converges input signals receives from multiple upstream and downstream signalling pathways either in the form of direct or indirect phosphorylation by multiple independent kinases and through its interactions with different proteins. In addition, inactivation of DAF-16 by AKT through phosphorylation can be further complicated because some isoforms like AKT-1 can have multiple (four) distinct phosphorylating sites that can produce more accessible and differential biological outputs [33, 34]. Furthermore, the C. elegans homolog of 5' AMP-activated protein kinase (AMPK) known as AAK-2 was engrossed in DAF-16/ FOXO stimulation and can enhance lifespan at the time of glucose limitation as reported [35]. AMPK is reported to have at least six different phosphorylating sites on DAF-16. By its inhibitory effects on the target of rapamycin (TOR) signalling, AMPK can also indirectly regulate DAF-16 activity. This illustrates how an organism's nutritional status is controlled through these different and highly conserved pathways. For example, in C. elegans, the TORC2 complex can activate serum and glucocorticoid-regulated kinase 1 (SGK-1), and its inhibition by AMPK should likely results in the activation of DAF-16 signalling [36, 37]. Therefore, both direct and indirect regulation of DAF-16 signalling can be possible through its crosstalk with AMPK signalling.
Direct interaction of the well-conserved c-Jun N-terminal kinase (JNK) signalling cascade with DAF-16 has also been reported, which has been linked to its lifespan extension activity and stress responses in worms [38]. This is possible because JNK can also have specific interaction sites for phosphorylation of DAF-16 that is different from that of AKT [39]. Integration of diverse inputs and unrelated signalling pathways like the Wingless and Int-1 (Wnt)/ βeta-catenin signalling pathway and germline signalling with DAF-16 have also been reported [40]. Furthermore, it has been reported that DAF-16 can also be regulated by processes like acetylation, dephosphorylation, proteasomal degradation, and other additional cofactors similar to mammalian FOXO [41]. These complex molecular cascades and different regulations could orchestrate/trigger transcription factor levels and the location of DAF-16/FOXO. Consecutively, other genes taking part in modulating growth, life expectancy, stress response, and metabolism gets activated or repressed [31, 42]. Thus, DAF-16 as a molecule represents itself as the chief life span controller in nematodes and is anticipated to react to different probiotics strains, including lactic acid bacteria.
Mitogen-Activated Protein Kinases
The evolutionary high conserved mitogen-activated protein kinases (MAPKs) have been linked with organism immunity and in the regulation of cellular responses to microbial and abiotic stresses [43, 44]. The two MAPK family members in C. elegans, namely the p38 MAPK and JNK pathways, are vital for organism homeostasis through their roles in activating basal and innate immune responses, oxidative stress and detoxification process [19].
p38 MAPK Pathway
Oxidative stress and other signals result in activation of PMK-1, which is a C. elegans ortholog of p38 MAPK. The worm PMK-1 is activated by its upstream MAP kinases known as neuronal symmetry-1 (NSY-1) and SAPK/ERK kinase-1 (SEK-1) [44, 45]. The p38 MAPK pathway is also reported to have enhancing activity for the worms' immune response in a unique way that is different from that of DAF-16 through its activation of another transcription factor known as skinhead-1 (SKN-1) [19, 46]. Similarly, under enhanced oxidative stress conditions, phosphorylation of SKN-1 at Ser-74 and Ser-340 position by PMK-1 has been reported in worms, and fluorescence intensity of SKN-1 green fluorescent protein (GFP) declined significantly with mutations as observed in skn-1(zu67) mutants. Additionally, when these phosphorylation sites are replaced with alanine, SKN-1 failed to translocate to the nuclei or rescue the prevailing oxidative stress [47]. The p38 MAPK pathway was recently linked to C. elegans immune responses when worms were fed with Lactobacillus acidophilus (L. acidophilus) [48]. In another study, when fed with Bifidobacterium infantis (B. infantis), improvement in the lifespan of C. elegans was observed in a dose-dependent manner through activation of skn-1 [49].
SKN-1 as a Transcription Factor for Oxidative Stress
In C. elegans, the transcription factor SKN-1 is orthologous to the basic-leucine zipper proteins in mammals known as Nrf1/2/3 (NF-E2-related factor). Recently, SKN-1 has been found to have pleiotropic effects on animal health because of its crucial involvement with multiple signalling inputs and its integrative roles concerning the phase II detoxification process and oxidative stress responses in C. elegans [50]. SKN-1 expression is predominant in the intestinal chemosensory neurons ASI; and its nuclear localization is necessary for the constitutive induction of phase II gene expressions. As part of an organism response, enhanced oxidative stress directs SKN-1 to delocalize and accumulate in nuclei for transactivating sets of genes necessary for defense and detoxification. Further, it has been confirmed wherein, the skn-1 mutant worms exhibit sensitivity to oxidative stress with a 25–30% shortened lifespan. SKN-1 as a transcription factor can directly integrate with multiple regulatory signals. In worms fed with Lactobacillus gasseri SBT2055 (LG2055), SKN‐1 activation has been reported, and this, in turn, regulate/enhances the transcriptional activity of various antioxidant genes (gst‐4, sod‐1, gcs‐1, clk‐1, trx‐1, hsp‐70 and hsp16.2) in comparison to E. coli OP50 fed worms [51]. SKN-1 also activates broad detoxification and xenobiotic genes. This ranges from a detoxification enzyme glutathione-S-transferase 4 (gst-4), lipid metabolism genes, and various genes involved in proteasomal activity, membrane and lysosomal functions necessary for the maintenance of homeostasis in an organism [52]. It was also well known that the JNK and p38/Erk MAPK pathways are potent positive regulators of SKN-1 [47, 52]. Glycogen synthase kinase 3 (GSK-3β) and SGK-1 signalling pathways enhance its phosphorylation and cytosolic localization, thereby negatively regulating SKN-1. Direct inhibition and prevention of SKN-1 accumulation in intestinal nuclei by IIS signalling in C. elegans have also been reported [46]. SKN-1 activity is also regulated by proteasomal degradation, and in C. elegans a protein named WD repeat protein 23 (WDR-23) acts analogously by targeting SKN-1 for degradation thus preventing its accumulation [53].
JNK Family
As a subgroup of the MAPK superfamily, the JNK family transduces signalling events in response to different inflammatory signals such as cytokines, including TNF and IL-1, and cues from prevailing environmental conditions. Several orthologs of JNK pathway components have been identified in C. elegans, which include: jnk-1 (a mammalian JNK orthologs), jkk-1 and mek-1 (orthologs of two JNK kinase, MKK4/7), and unc-16 (JIP3 scaffold protein orthologs) [54, 55]. In a recent study, JNK and p38/PMK-1 were evaluated to determine their involvement in DAF-16-dependent lifespan extension as seen induced by Bifidobacterium longum BB68 (BB68) strain. BB68 could potentially increase the lifetime of pmk-1 (km25) mutants as opposed to the strain of worms with a mutation in jnk-1 gene (gk7), where only the activated JNK-1 was seen in the nerve ring of nematodes fed with BB68. Besides, the nuclear localization of DAF-16, mediated through BB68 strain, was also reduced in jnk-1 mutant worms [32]. The JNK pathway has been found to have direct physical interaction with DAF-16; it can positively promote its translocation to the nucleus and, thus, is involved in DAF-16 mediated lifespan extension and heat stress tolerance in worms [38].
TGF-β Pathway
The Transforming Growth Factor-β (TGF-β) represents another ancient superfamily of pathways necessary for maintaining homeostasis through intercellular communications and signal amplification in eukaryotic animals. In C. elegans, there are five TGF-β-related genes identified, and the conventional TGF-β pathway is also referred to as the Sma/Mab pathway because mutations in this pathway can produce worms with a phenotype of the small body (Sma phenotype), and defective tails in male worms (Mab phenotype). Its expressions is mostly seen in the subset of neurons like pharyngeal neurons. The binding of pathway ligand DBL-1 to the Type I/II receptors SMA6 and DAF-4 is necessary for activation of the downstream effector SMADs SMA-2, − 3 and − 4, which functions together with the Schnurri homolog SMA-9 to upregulate or downregulate sets of target genes in C. elegans [56]. TGF-β signalling pathway is crucial for regulating longevity, cell migration and patterning, body size, chemosensation, and neuronal functions besides other roles like immune and behavioural responses. Recently two good probiotics Butyricicoccus pullicaecorum (B. pullicaecorum) and Megasphaera elsdenii (M. elsdenii), were reported to have extended the lifespan of C. elegans via the TGF-β pathway [57].
Effects of Probiotics in C. elegans
Lifespan Extension
The aging process was associated with changes in intestinal microbial composition, which is frequently connected with alterations in the digestive tract and the long-term effects of food habits. Thus, a dietary pattern that helps re-establish the microbiota in older may help reduce the frailty and weakness besides broadening the health span of an individual. Investigations on C. elegans have shown positive effects concerning health and the lifespan of nematodes nourished with certain strains of lactobacilli. The outcomes hugely relied on specific strain fed and its specific aspects like multiplying potentiality. Feeding of C. elegans with various bacterial strains in the standard laboratory conditions has also been reported to produce substantial dissimilarities in life-history characteristics like longevity, fecundity, and its development rate. For example, when Comamonas aquatic (C. aquatica)- DA1877 (DA1877) was supplied as a diet to C. elegans, accelerated growth, reduced fecundity, and a shorter lifetime was observed as compared to animals fed with E. coli OP50. On the contrary, when worms were fed with a low quantity of DA1877 diluted with E. coli OP50 (1:1000), developmental acceleration and reduced fecundity were seen [58]. This study reveals that DA1877 can generate a dilatable metabolic substance that can modulate the nematodes' life-history characteristics. Similarly, in another study highlighting the effect of distinct bacterial strains derived from the natural environment on C. elegans, from the whole lot examined, 80% displayed robust development of the animal while 20% exhibit debilitated growth where stress reporter was instigated [59].
In another interesting study, the worms' enhanced survival rate was reported after worms were exposed to heat stress at 35 °C before hydrogen peroxide (H2O2) induced oxidative stress when fed with either heat-killed Bifidobacterium longum (B. longum) (5·0 mg/ml) and/or E. coli OP50. Pro-longevity and enhanced mobility were also observed in the nematodes provided with the feed of killed B. longum compared to those served with only E. coli. OP50. Furthermore, nuclear translocation of DAF-16 and the upregulation of the DAF-16 target gene hsp-12.6 were reported. Such observations elucidate the functional impacts of killed B. longum in the nematodes through stimulation of DAF-16 [60]. Lee and his colleagues reported that when Weissella koreensis (W. koreensis) and Weissella cibaria (W. cibaria) (20 mg wet weight) were fed to C. elegans grown in a 90-mm diameter plate, a substantial improvement in worm’s lifespan was observed in comparison to worms fed with E. coli OP50, and this is linked to the upregulation of several genes including aak-2, daf-16, jnk-1, hif-1, and sod-3, which are connected with lifespan extension. Additionally, even among the same species, worms fed with W. cibaria upregulated all the genes mentioned above, except daf-2, by more than two-fold. In particular, daf-16 and sod-3 were upregulated by 3.9-fold and 2.3-fold, respectively, compared to the control group fed with E. coli OP50. These findings illustrate these genes' pivotal roles in extending the lifespan of worms supplied with Weissella species [61].
Investigation on the impact of B. infantis on the life expectancy of C. elegans had identified a reasonable dose-dependent lifespan augmentation when B. infantis was supplemented along with E. coli OP50. Moreover, an increase in the health span of nematodes served with B. infantis corroborates with its physiological and mechanical parameters of enhanced mobility and coordination observed. Simultaneously, when the cell wall or protoplast portions of B. infantis were assigned as the diet to C. elegans, those similar probiotic effects were noticed, pinpointing to the immune-modulatory effects of lipoteichoic acid that is present in its cell wall as well as protoplast portions abundantly. Furthermore, B. infantis served nematodes displayed lesser aggregation of the self-fluorescent pigment namely lipofuscin, popularly employed as an indicator for aging in C. elegans. In-depth molecular studies on the effects of B. infantis using mutant strains of C. elegans revealed that the bacterium's influence towards longevity is ascribed to the SKN-1 stimulation by the p38 MAPK pathway [49].
The lifespan extension benefit of a particular strain of probiotics can also be linked to its caloric value/limitations when consumed. For example, Lactobacillus salivarius strain FDB89 (FDB89), isolated from centenarian faeces was also reported for longevity effect with the help of C. elegans. This study estimates longevity, reproductive ability, body size, SOD activity, and XTT (2,3-bis-(2-methoxy-4nitro-5-sulfophenyl-2H-tetrazolium-5-carboxanilide) reduction capacity, and pharyngeal pumping were evaluated after supplementing FDB89 strain with an ordinary laboratory diet of C. elegans. The study uncovered that FDB89 exhibits lifespan extension benefits of about 11.9% compared to the control group but decelerated the development, pharyngeal pumping, and reproductive ability of the worms. Also, enhanced XTT reduction capacity and SOD activity were observed. It was assumed that up to 10 times increase in lifespan was contributed through caloric restriction, as per the gradient feeding assay, in contrast with the standard measure of nourishment. The FDB89 addition in the diet has not changed the lifespan of eat-2 (gene that controls pharyngeal pumping) mutant worms, indicating that lifespan extension in N2 worms was due to FDB89 consumption, and the mechanism rely upon the caloric-limitation [62].
In another comparative study, alterations in longevity were examined using N2 and mutant strains of C. elegans after feeding with E. coli OP50, Bacillus licheniformis (B. licheniformis) and Lactobacillus rhamnosus GG (L. rhamnosus) (20 μL aliquots of concentrated bacteria plated on 35 mm diameter NGM agar plates). B. licheniformis broadened the lifespan of the nematode by 45% in comparison to E. coli OP50 control. The colonizing capacity of B. licheniformis in the intestine of the nematode was found to be lesser than that of E. coli OP50 and L. rhamnosus. Further investigations with mutant strains for bas-1 (serotonin-and dopamine-engineered aromatic amino acid decarboxylase), ser-1, and ser-7 (serotonin receptor), mod-1 (serotonin-gated chloride channel), tph-1 (tryptophan hydroxylase) gene uncovered that B. licheniformis affects serotonin signal transduction framework. The pro-longevity has interceded via the serotonin signal transduction [63].
Pathogen Resistance
Around the world, bacterial contagions remain a substantial source of sickness and fatality. Excessive antibiotic usage sparked off antibiotic resistance, microbiome depletion that was harmful to the individual, leading to communal health problems. Treatment of contagious diseases by dint of probiotics remains an improvised approach to halt bacteria's growth exhibiting multiple resistances. On the research front of microbial resistance using probiotics, mammalian model systems face challenges such as ethical constraints, diverse network of microbes, inconsistent host hereditary foundation, and impacts of food intake. On the other hand, C. elegans imparts a hereditarily compatible framework for investigating the effect of the desired species of bacteria. For example, when worms were grown (25 °C for 24 h) and fed with L. acidophilus as a food source, it rendered them with a defensive impact against the Gram-positive harmful bacteria such as Enterococcus faecalis (E. faecalis) and Staphylococcus aureus (S. aureus), yet by no means on those belonging to Gram-negative group like Pseudomonas aeruginosa (P. aeruginosa) [48]. On further evaluation of C. elegans tir-1, bar-1, and pmk-1 mutants demonstrate that the Toll/interleukin-1 receptor, the β-catenin signalling pathway, and the p38 MAP kinase pathway are associated with L. acidophilus-interceded security of worms against Gram-positive pathogens. Microbe-associated molecular patterns (MAMPs) or damage-associated molecular patterns (DAMPs), which help modulate pathogen-defensive host pathways, were also highlighted. The changes mentioned above cannot be established in C. elegans pmk-1 mutants, reiterating that the defensive of worms is mediated using the p38 MAPK pathway [64].
Many bacterial diets were also found to have a protective effect against microbes' inherent ability to cause infections. For example, C. elegans were grown and fed with B. subtilis GS67 (GS67) (40 ml was spread on 6 cm NGM plates) and incubated overnight, it exhibited firm defiance to the morbific impacts of Bacillus thuringiensis DB27 (DB27). GS67 directly inhibits the proliferation of DB27 and also minimizes the inhabitation of DB27 in the gut of C. elegans. A GS67 mutant, deficient in fengycin synthesis lacks protection against DB27 and was unfit to safeguard the worm out of DB27, reflecting the significance of fengycin in suppressing pathogens and in C. elegans protection [65]. Similarly, C. elegans fed with the diet combination of B. megaterium and P. mendocina have shown protective effects against pathogenicity of P. aeruginosa in comparison to worms raised on E. coli OP50. B. megaterium and P. mendocina induces C. elegans resilience to infection over apparent mechanisms. The propensity of B. megaterium to augment infection resilience was connected to its impacts on reproduction, whereas P. mendocina executes by modulating the p38-dependent MAP kinase pathway that serves as the innate immune system of the worms [64]. Elsewhere, it has also been reported that Bacillus subtilis NCIB3610, which can form a biofilm, helps in nematode longevity by upregulation of lys-2 expression and in intensifying C. elegans resilience to P. aeruginosa infection [66].
Probiotic-mediated switching on of the discrete host pathways, especially the p38 MAPK pathway, could contribute moderately to safeguarding against contagious microbes. For example, a study revealed a heightened resistance of worms to E. coli O127: H6, which lasted up to 24 h, depending upon the p38 MAPK and ILS pathways, accompanied by neurons synthesizing dopamine [67]. Different researches and reviews questions on the sole role of DAF-16 in probiotic-intervened lifespan expansion, and that, different self-directed pathways are also involved in C. elegans longevity [68, 69]. While preceding models of host-microbe relations have strived to change the host microbiota's intricacy, many novel and dynamic techniques have also helped in further understanding of the collective symbiosis which takes place in a host system. For example, recently, researches exploring horizontal gene transfer among bacteria inside the intestine of nematode and meta-analysis of C. elegans microarray have highlighted differential gene expressions in the period of a contagious illness, inhabitation, or proliferation of C. elegans on a wide range of microorganisms [70, 71].
Stress Resistance
Prolonged exposure to oxidative stress is known to have deleterious effects on various organisms, and antioxidant barriers safeguard cells by eliminating reactive oxygen species (ROS). On the contrary, during the aging process and in diseased conditions, ROS and other oxygen metabolism compounds accumulate. Their toxicity disturbs the genetic material, fats, proteins, and antioxidant barrier systems. Thus, increased lifespan is often correlated with enhanced resistance against oxidative damage. Amid the explored strains, Bifidobacterium longum strain BR-108 has been reported to have improved C. elegans longevity after the H2O2-induced oxidative stress by modulation of IIS pathway. DAF-16 appears to localize within the nucleus coupled with the heat-shock transcription factor (HSF)-1, stimulating the transcription of hsp-16.2 and hsp-70 that are implicated in lifespan extension and stress responses [60]. Similarly, Lactobacillus rhamnosus CNCM I-3690 and Bifidobacterium animalis subsp. lactis CECT8145 strains were reported to have heightened oxidative stress resistance in the nematode, which was partly linked with the involvement of IIS pathway [72, 73]. Analyzing the impact of resistance against temperature-mediated stress in the nematode revealed that thermal stress resistance of worms served with LG2055 was greater when compared to worms fed with E. coli OP50.
Worms fed with LG2055 showed significant oxidative stress tolerance (stress resistance for 16 h following exposure to paraquat) instead of worms served with E. coli OP50, where 40% deemed dead 2 h following exposure to paraquat. Additionally, LG2055 even enhances the longevity of short-lived Methyl viologen-sensitive gene (mev-1) mutants that are deficient in succinate coenzyme Q oxidoreductase (a component of complex II of the electron transport system in the mitochondria) that are characterized with elevated levels of intrinsic oxidative stress due to higher superoxide anion formation [20, 74]. These findings highlighted the significant role of probiotics in counteracting intrinsic oxidative stress, especially in mitochondria, which are closely linked with the aging process. Other examples of the different biological effects of probiotics elucidated through C. elegans model are shown in Table 1.
Table 1.
Biological effects of some probiotics in C. elegans model
| Subject of investigation | Probiotic strains | Findings | Relative genes/pathway studied | Reference |
|---|---|---|---|---|
| Immune-modulation | Enterococcus faecium L11 | Upregulate genes for host defence, protective effect against Salmonella Typhimurium infection in C. elegans |
TGF-β pathway (dbl-1 and sma-3) MAPK pathway (pmk-1 and sek-1) |
[79] |
| Proteotoxicity | Bacillus subtilis PXN21 | Inhibit, delay and reverses α-synuclein aggregation in C. elegans | Daf-16, sphingolipid metabolism pathway (lagr-1, asm-3, and sptl-3) | [80] |
| Energy and lipid metabolism | Lactobacillus delbrueckii, L. fermentum, and Leuconostoc lactis | Alter the expression of genes associated with obesity phenotypes in C. elegans | nhr-49, pept-1, and tub-1 | [81] |
| Cancer Chemotherapeutics | Lactobacillus reuteri, Lactobacillus salivarius, Pediococcus acidilactici | Inhibit the growth of tumour-like germ cells | gld-1 | [82] |
| E. coli BW25113, Comamonas aquatica DA1877 | Understanding the complexity of host-microbe-drug interactions | ndk-1 | [83, 84] | |
| Toxicology | Lactobacillus bulgaricus | Protection from graphine oxide toxicity to both primary and secondary targeted organs of C. elegans | acs-22 | [85] |
| Behavioural | Bifidobacterium infantis | Involved in leaving behaviour in worms | tol-1 | [86] |
| Antifungal | Lactobacillus rhamnosus Lcr35 | Mechanistic insights against Candida albicans infection using C. elegans | p38 MAPK signalling pathway | [87] |
| Cell signalling | Bifidobacterium infantis | Involvement of Toll like receptor (TLR) in leaving behaviour in C. elegans | tol-1 | [86] |
Future Prospective
Mechanisms underlying various host-specific regulatory roles by members of the microbiome required further investigations. On the same front, there is no versatile probiotic strain that functions efficiently for every person since the intestinal microbiota varied across mankind. Thus, novel approaches in the form of microbial cocktails as targets to restore dysbiosis states in human diseases can be thought of. Unraveling pathways that regulate the microbiome using innovative C. elegans sensitive screening platforms and high throughput approaches will uncover valuable information relevant to higher organisms and also in the understanding of different mechanisms of microbial ecosystem succession. Moreover, approaches in the form of using disease-specific microbes humanized in C. elegans will help devise strategies for targeted therapeutics [75]. With the advancement of artificial intelligence, metabolic engineering, synthetic biology alongside the applications of various genome editing tools, the designing of probiotics may represent exciting avenues of structuring organisms to target explicit tissues and cells as opposed to the entire body and in the production of novel probiotics with wanted attributes and capabilities [76–78]. Does the hindering of pathogenic microorganisms by designing/engineered probiotics represents a promising approach for maintaining gastrointestinal wellbeing and in treatment of bacterial diseases? The answer lies in the future. Needlessly, the role of C. elegans as a model organism in probiotics research of the future is expected to have a huge impact right from the screening process and in the elucidation of the different molecular mechanisms involved in the process.
Conclusion
C. elegans as an in vivo model organism have been utilized as a sensitive screening platform for new probiotics and in monitoring their interactions with the host system. The exclusive feeding property of C. elegans on bacteria has simplified probiotics researches wherein, easy and economical manipulations of its diet can be carried out to analyze promising probiotic strains from a huge collection of microbes. At the molecular level, studies on C. elegans have revealed that different probiotic strains have the capacity of exerting their beneficial effects through their regulatory roles on conserved signalling pathways like p38MAP kinase and Insulin/IGF-1 (IIS) pathway. Studies from C. elegans have also shown that transcription factors like DAF-16, SKN-1, and HSF-1 can be used as sensitive targets/markers for elucidating differential probiotics effects on the host organism. Nonetheless, novel probiotic strains and additional essential factors ought to be discovered for safe and target specific suitable in dietary management regimen for human wellbeing. Futuristic approaches in the form of differential gene expressions from microarray data and synthetic biology is expected to speed-up the identification process of novel natural/engineered probiotics. In this perspective, C. elegans appears to be advantageous for shedding light on key mechanisms involved in host response to probiotics supplementation and in pinpointing of candidate probiotic strain necessary for ‘good’ gut microbiome.
Acknowledgements
The authors acknowledge the Department of Biochemistry and Molecular Biology, Pondicherry University and Department of Science & Technology (DST), India for financial and overall support.
Author Contributions
RM: writing, review, and editing. PI: writing, review and editing. DN: review and editing. KS: writing, review, and editing.
Declaration
Conflict of interest
The authors declare that they have no conflict of interest. All authors gave informed consent to the submission of this manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G. Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc Natl Acad Sci USA. 2009;106:3698–3703. doi: 10.1073/pnas.0812874106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackowiak PA. Recycling Metchnikoff: probiotics, the intestinal microbiome and the quest for long life. Front Public Health. 2013;1:52. doi: 10.3389/fpubh.2013.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song H, Zhou L, Liu D, Ge L, Li Y. Probiotic effect on Helicobacter pylori attachment and inhibition of inflammation in human gastric epithelial cells. Exp Ther Med. 2019;18:1551–1562. doi: 10.3892/etm.2019.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Askarova S, Umbayev B, Masoud AR, Kaiyrlykyzy A, Safarova Y, Tsoy A, Olzhayev F, Kushugulova A. The links between the gut microbiome, aging, modern lifestyle and Alzheimer's disease. Front Cell Infect Microbiol. 2020;10:104. doi: 10.3389/fcimb.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel P, Singh SK, Panaich S, Cardozo L. The aging gut and the role of prebiotics, probiotics, and synbiotics: a review. J Clin Gerontol Geriatr. 2014;5:3–6. [Google Scholar]
- 6.Taverniti V, Scabiosi C, Arioli S, Mora D, Guglielmetti S. Short-term daily intake of 6 billion live probiotic cells can be insufficient in healthy adults to modulate the intestinal bifidobacteria and lactobacilli. J Funct Foods. 2013;6:482–491. [Google Scholar]
- 7.Fijan S. Microorganisms with claimed probiotic properties: an overview of recent literature. Int J Environ Res Public Health. 2014;11:4745–4767. doi: 10.3390/ijerph110504745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding RX, Goh WR, Wu RN, Yue XQ, Luo X, Khine WWT, Wu JR, Lee YK. Revisit gut microbiota and its impact on human health and disease. J Food Drug Anal. 2019;27:623–631. doi: 10.1016/j.jfda.2018.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Porras AM, Brito IL. The internationalization of human microbiome research. Curr Opin Microbiol. 2019;50:50–55. doi: 10.1016/j.mib.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurung M, Li Z, You H, Rodrigues R, Jump DB, Morgun A, Shulzhenko N. Role of gut microbiota in type 2 diabetes pathophysiology. EBio Med. 2020;51:102590. doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanz Y, Rastmanesh R, Agostonic C. Understanding the role of gut microbes and probiotics in obesity: how far are we? Pharmacol. Res. 2013;69:144–155. doi: 10.1016/j.phrs.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Asemi Z, Samimi M, Tabassi Z, Naghibi Rad M, Rahimi Foroushani A, Khorammian H, Esmaillzadeh A. Effect of daily consumption of probiotic yoghurt on insulin resistance in pregnant women: a randomized controlled trial. Eur J Clin Nutr. 2013;67:71–74. doi: 10.1038/ejcn.2012.189. [DOI] [PubMed] [Google Scholar]
- 13.Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28:539–543. doi: 10.1016/j.nut.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 14.Ooi LG, Liong MT. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. 2010;11:2499–2522. doi: 10.3390/ijms11062499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma J, Li H. The role of gut microbiota in atherosclerosis and hypertension. Front Pharmacol. 2018;9:1082. doi: 10.3389/fphar.2018.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsieh FC, Lee CL, Chai CY, Chen WT, Lu YC, Wu CS. Oral administration of Lactobacillus reuteri GMNL-263 improves insulin resistance and ameliorates hepatic steatosis in high fructose-fed rats. Nutr Metab (Lond) 2013;10:35. doi: 10.1186/1743-7075-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cionci NB, Baffoni L, Gaggìa F, Di Gioia D. Therapeutic microbiology: the role of bifidobacterium breve as food supplement for the prevention/treatment of paediatric diseases. Nutrients. 2018;10:1723. doi: 10.3390/nu10111723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 19.Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006;2:e183. doi: 10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa H, Shiozaki T, Kobatake E, Hosoya T, Moriya T, Sakai F, Taru H, Miyazaki T. Effects and mechanisms of prolongevity induced by Lactobacillus gasseri SBT2055 in Caenorhabditis elegans. Aging Cell. 2016;15:227–236. doi: 10.1111/acel.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sin O, Michels H, Nollen EA. Genetic screens in Caenorhabditis elegans models for neurodegenerative diseases. Biochim Biophys Acta. 2014;1842:1951–1959. doi: 10.1016/j.bbadis.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 22.O’Reilly LP, Knoerdel RR, Silverman GA, Pak SC. High-throughput, liquid-based genome-wide RNAi screening in C. elegans. Methods Mol Biol. 2016;1470:151–162. doi: 10.1007/978-1-4939-6337-9_12. [DOI] [PubMed] [Google Scholar]
- 23.Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, Ruvkun G. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- 24.Hillier LW, Coulson A, Murray JI, Bao Z, Sulston JE, Waterston RH. Genomics in C. elegans: So many genes, such a little worm. Genome Res. 2005;15:1651–1660. doi: 10.1101/gr.3729105. [DOI] [PubMed] [Google Scholar]
- 25.Berg M, Stenuit B, Ho J, Wang A, Parke C, Knight M, Alvarez-Cohen L, Shapiraet M. Assembly of the Caenorhabditis elegans gut microbiota from diverse soil microbial environments. ISME J. 2016;10:1998–2009. doi: 10.1038/ismej.2015.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dirksen P, Marsh SA, Braker I, Heitland N, Wagner S, Nakad R, Mader S, Petersen C, Kowallik V, Rosenstiel P, Félix MA, Schulenburg H. The native microbiome of the nematode Caenorhabditis elegans: Gateway to a new host-microbiome model. BMC Biol. 2016;14:38. doi: 10.1186/s12915-016-0258-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coolon JD, Jones KL, Todd TC, Carr BC, Hermanet MA. Caenorhabditis elegans genomic response to soil bacteria predicts environment-specific genetic effects on life history traits. PLoS Genet. 2009;5:e1000503. doi: 10.1371/journal.pgen.1000503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurz CL, Ewbank JJ. Caenorhabditis elegans: an emerging genetic model for the study of innate immunity. Nat Rev Genet. 2003;4:380–390. doi: 10.1038/nrg1067. [DOI] [PubMed] [Google Scholar]
- 29.Zugasti O, Ewbank JJ. Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-β signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol. 2009;10:249–256. doi: 10.1038/ni.1700. [DOI] [PubMed] [Google Scholar]
- 30.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell. 1999;96:857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 31.Mukhopadhyay A, Oh SW, Tissenbaum HA. Worming pathways to and from DAF-16/FOXO. Exp Gerontol. 2006;41:928–934. doi: 10.1016/j.exger.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L, Zhao Y, Liu R, Zheng X, Zhang M, Guo H, Zhang H, Renet F. The transcription factor DAF-16 is essential for increased longevity in C elegans exposed to Bifidobacterium longum BB68. Sci Rep. 2017;7:7408. doi: 10.1038/s41598-017-07974-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28:139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 34.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 35.Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Current Biology. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narasimhan SD, Yen K, Tissenbaum HA. Converging pathways in lifespan regulation. Curr Biol. 2009;19:R657. doi: 10.1016/j.cub.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G. Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev. 2009;23:496–511. doi: 10.1101/gad.1775409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh SW, Mukhopadhyay A, Svrzikapa N, Iang F, Davis RJ, Tissenbaum HA. JNK regulates lifespan in Caenorhabiditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. Proc Natl Acad Sci U S A. 2005;102:4494–4499. doi: 10.1073/pnas.0500749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Essers MA, Weijzen S, De Vries-Smits AM, Saarloos I, de Ruiter ND, Bos JL, Burgering BM. FOXO transcription factor activation by oxidative stress mediated by the small GTPase Ral and JNK. EMBO J. 2004;23:4802–4812. doi: 10.1038/sj.emboj.7600476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoogeboom D, Essers MA, Polderman PE, Voets E, Smits LM, Burgering BM. Interaction of FOXO with β-catenin inhibits β-catenin/T cell factor activity. J Biol Chem. 2008;283:9224–9230. doi: 10.1074/jbc.M706638200. [DOI] [PubMed] [Google Scholar]
- 41.Van Der Heide LP, Hoekman MFM, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004;380:297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 43.Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. Map kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415:977–983. doi: 10.1038/415977a. [DOI] [PubMed] [Google Scholar]
- 44.Dong J, Chen C, Chen Z. Expression profiles of the Arabidopsis WRKY gene superfamily during plant defense response. Plant Mol Biol. 2003;51:21–37. doi: 10.1023/a:1020780022549. [DOI] [PubMed] [Google Scholar]
- 45.Sakaguchi A, Matsumoto K, Hisamoto N. Roles of MAP kinase cascades in Caenorhabditis elegans. J Biochem. 2004;136:7–11. doi: 10.1093/jb/mvh097. [DOI] [PubMed] [Google Scholar]
- 46.Hoeven Rv, McCallum KC, Cruz MR, Garsin DA. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p 38 MAPK signaling during infection in C. elegans. PLoS Pathog. 2011;7:1002453. doi: 10.1371/journal.ppat.1002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inoue H, Hisamoto N, Jae HA, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19:2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y, Mylonakis E. Caenorhabditis elegans immune conditioning with the probiotic bacterium Lactobacillus acidophilus strain ncfm enhances gram-positive immune responses. Infect Immun. 2012;80:2500–2508. doi: 10.1128/IAI.06350-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komura T, Ikeda T, Yasui C, Saeki S, Nishikawa Y. Mechanism underlying prolongevity induced by bifidobacteria in Caenorhabditis elegans. Biogerontology. 2013;14:7–87. doi: 10.1007/s10522-012-9411-6. [DOI] [PubMed] [Google Scholar]
- 50.An JH, Blackwell TK. SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev. 2003;17:1882–1893. doi: 10.1101/gad.1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliveira RP, Abate JP, Dilks K, Landis J, Ashraf J, Murphy CT, Blackwell TK. Condition-adapted stress and longevity gene regulation by Caenorhabditis elegans SKN-1/Nrf. Aging Cell. 2009;8:524–541. doi: 10.1111/j.1474-9726.2009.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okuyama T, Inoue H, Ookuma S, Satoh T, Kano K, Honjoh S, Hisamoto N, Matsumoto K, Nishida E. The ERK-MAPK pathway regulates longevity through SKN-1 and insulin-like signaling in Caenorhabditis elegans. J Biol Chem. 2010;285:30274–30281. doi: 10.1074/jbc.M110.146274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choe KP, Przybysz AJ, Strange K. The WD40 repeat protein WDR-23 functions with the CUL4/DDB1 ubiquitin ligase to regulate nuclear abundance and activity of SKN-1 in Caenorhabditis elegans. Mol Cell Biol. 2009;29:2704–2715. doi: 10.1128/MCB.01811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villanueva A, Lozano J, Morales A, Lin X, Deng X, Hengartner MO, Kolesnick RN. jkk-1 and mek-1 regulate body movement coordination and response to heavy metals through jnk-1 in Caenorhabditis elegans. EMBO J. 2001;20:5114–5128. doi: 10.1093/emboj/20.18.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byrd DT, Kawasaki M, Walcoff M, Hisamoto N, Matsumoto K, Jin Y. UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron. 2001;32:787–800. doi: 10.1016/s0896-6273(01)00532-3. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki Y, Yandell MD, Roy PJ, Krishna S, Savage-Dunn C, Ross RM, Padgett RW, Wood WB. A BMP homolog acts as a dose-dependent regulator of body size and male tail patterning in Caenorhabditis elegans. Development. 1999;126:241–250. doi: 10.1242/dev.126.2.241. [DOI] [PubMed] [Google Scholar]
- 57.Kwon G, Lee J, Koh JH, Lim YH. Lifespan Extension of Caenorhabditis elegans by Butyricicoccus pullicaecorum and Megasphaera elsdenii with probiotic potential. Curr Microbiol. 2018;75:557–564. doi: 10.1007/s00284-017-1416-6. [DOI] [PubMed] [Google Scholar]
- 58.MacNeil LT, Watson E, Arda HE, Zhu LJ, Walhout AJ. Diet-induced developmental acceleration independent of TOR and insulin in C. elegans. Cell. 2013;153:240–252. doi: 10.1016/j.cell.2013.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Samuel BS, Rowedder H, Braendle C, Félix MA, Ruvkun G. Caenorhabditis elegans responses to bacteria from its natural habitats. Proc Natl Acad Sci U S A. 2016;113:E3941–E3949. doi: 10.1073/pnas.1607183113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugawara T, Sakamoto K. Killed bifidobacterium longum enhanced stress tolerance and prolonged life span of Caenorhabditis elegans via DAF-16. Br J Nutr. 2018;120:872–880. doi: 10.1017/S0007114518001563. [DOI] [PubMed] [Google Scholar]
- 61.Lee J, Kwon G, Lim YH. Elucidating the mechanism of Weissella-dependent lifespan extension in Caenorhabditis elegans. Sci Rep. 2015;5:17128. doi: 10.1038/srep17128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y, Zhao L, Zheng X, Fu T, Guo H, Ren F. Lactobacillus salivarius strain FDB89 induced longevity in Caenorhabditis elegans by dietary restriction. J Microbiol. 2013;51:183–188. doi: 10.1007/s12275-013-2076-2. [DOI] [PubMed] [Google Scholar]
- 63.Park MR, Oh S, Son SJ, Park DJ, Oh S, Kim SH, Jeong DY, Oh NS, Lee Y, Song M, Kim Y. Bacillus licheniformis isolated from traditional korean food resources enhances the longevity of Caenorhabditis elegans through serotonin signaling. J Agric Food Chem. 2015;63:10227–10233. doi: 10.1021/acs.jafc.5b03730. [DOI] [PubMed] [Google Scholar]
- 64.Montalvo-Katz S, Huang H, Appel MD, Berg M, Shapira M. Association with soil bacteria enhances p38-dependent infection resistance in Caenorhabditis elegans. Infect Immun. 2013;81:514–520. doi: 10.1128/IAI.00653-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iatsenko I, Yim JJ, Schroeder FC, Sommer RJ. B. subtilis GS67 protects C. elegans from gram-positive pathogens via fengycin-mediated microbial antagonism. Curr Biol. 2014;24:2720–2727. doi: 10.1016/j.cub.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 66.Smolentseva O, Gusarov I, Gautier L, Shamovsky I, DeFrancesco AS, Losick R, Nudler E. Mechanism of biofilm-mediated stress resistance and lifespan extension in C. elegans. Sci Rep. 2017;7:7137. doi: 10.1038/s41598-017-07222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Anyanful A, Easley KA, Benian GM, Kalman D. Conditioning protects C. elegans from lethal effects of enteropathogenic E. coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe. 2009;5:450–462. doi: 10.1016/j.chom.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roselli M, Schifano E, Guantario B, Zinno P, Uccelletti D, Devirgiliis C. Caenorhabditis elegans and probiotics interactions from a prolongevity perspective. Int J Mol Sci. 2019;20:5020. doi: 10.3390/ijms20205020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Engelmann I, Griffon A, Tichit L, Montañana-Sanchis F, Wang G, Reinke V, Waterston RH, Hillier LW, Ewbank JJ. A comprehensive analysis of gene expression changes provoked by bacterial and fungal infection in C elegans. PLoS One. 2011;6:e19055. doi: 10.1371/journal.pone.0019055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clark LC, Hodgkin J. Commensals, probiotics and pathogens in the Caenorhabditis elegans model. Cell Microbiol. 2014;16:27–38. doi: 10.1111/cmi.12234. [DOI] [PubMed] [Google Scholar]
- 71.Nicholson JK, Wilson ID. Understanding “global” systems biology: metabonomics and the continuum of metabolism. Nat Rev Drug Discov. 2003;2:668–676. doi: 10.1038/nrd1157. [DOI] [PubMed] [Google Scholar]
- 72.Grompone G, Martorell P, Llopis S, González N, Genovés S, Mulet AP, Fernández-Calero T, Tiscornia I, Bollati-Fogolín M, Chambaud I, Foligné B, Montserrat A, Ramón D. Anti-inflammatory Lactobacillus rhamnosus CNCM I-3690 strain protects against oxidative stress and increases lifespan in Caenorhabditis elegans. PLoS One. 2012;7:e52493. doi: 10.1371/journal.pone.0052493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martorell P, Llopis S, González N, Chenoll E, López-Carreras N, Aleixandre A, Chen Y, Karoly ED, Ramón D, Genovés S. Probiotic strain Bifidobacterium animalis subsp. lactis CECT 8145 reduces fat content and modulates lipid metabolism and antioxidant response in Caenorhabditis elegans. J Agric Food Chem. 2016;64:3462–3472. doi: 10.1021/acs.jafc.5b05934. [DOI] [PubMed] [Google Scholar]
- 74.Ishii N, Fujii M, Hartman PS, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa D, Suzuki K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394:694–697. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- 75.Silverman GA, Luke CJ, Bhatia SR, Long OS, Vetica AC, Perlmutter DH, Pak SC. Modeling molecular and cellular aspects of human disease using the nematode Caenorhabditis elegans. Pediatr Res. 2009;65:10–18. doi: 10.1203/PDR.0b013e31819009b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kumar P, Sinha R, Shukla P. Artificial intelligence and synthetic biology approaches for human gut microbiome. Crit Rev Food Sci Nutr. 2020;30:1–19. doi: 10.1080/10408398.2020.1850415. [DOI] [PubMed] [Google Scholar]
- 77.Yadav M, Mandeep SP. Probiotics of diverse origin and their therapeutic applications: a review. J Am Coll Nutr. 2020;39:469–479. doi: 10.1080/07315724.2019.1691957. [DOI] [PubMed] [Google Scholar]
- 78.Zhou Z, Chen X, Sheng H, Shen X, Sun X, Yan Y, Wang J, Yuan Q. Engineering probiotics as living diagnostics and therapeutics for improving human health. Microb Cell Fact. 2020;19:56. doi: 10.1186/s12934-020-01318-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sim I, Park KT, Kwon G, Koh JH, Lim YH. Probiotic potential of Enterococcus faecium isolated from chicken cecum with immunomodulating activity and promoting longevity in Caenorhabditis elegans. J Microbiol Biotechnol. 2018;28:883–892. doi: 10.4014/jmb.1802.02019. [DOI] [PubMed] [Google Scholar]
- 80.Goya ME, Xue F, Sampedro-Torres-Quevedo C, Arnaouteli S, Riquelme-Dominguez L, Romanowski A, Brydon J, Ball KL, Stanley-Wall NR, Doitsidou M. Probiotic Bacillus subtilis protects against α-Synuclein aggregation in C. elegans. Cell Rep. 2020;30:367–380. doi: 10.1016/j.celrep.2019.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zanni E, Laudenzi C, Schifano E, Palleschi C, Perozzi G. Impact of a complex food microbiota on energy metabolism in the model organism Caenorhabditis elegans. Biomed Res Int. 2015;2015:1–12. doi: 10.1155/2015/621709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fasseas MK, Fasseas C, Mountzouris KC, Syntichaki P. Effects of Lactobacillus salivarius, Lactobacillus reuteri, and Pediococcus acidilactici on the nematode Caenorhabditis elegans include possible antitumor activity. Appl Microbiol Biotechnol. 2013;97:2109–2118. doi: 10.1007/s00253-012-4357-9. [DOI] [PubMed] [Google Scholar]
- 83.Scott TA, Quintaneiro LM, Norvaisas P, Lui PP, Wilson MP, Leung KY, Herrera-Dominguez L, Sudiwala S, Pessia A, Clayton PT, Bryson K, Velagapudi V, Mills PB, Typas A, Greene NDE, Cabreiro F. Host-microbe co-metabolism dictates cancer drug efficacy in C. elegans. Cell. 2017;169:442–456. doi: 10.1016/j.cell.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.García-González AP, Ritter AD, Shrestha S, Andersen EC, Yilmaz LS, Walhout AJM. Bacterial metabolism affects the C. elegans response to cancer chemotherapeutics. Cell. 2017;169:431–441. doi: 10.1016/j.cell.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao Y, Yu X, Jia R, Yang R, Rui Q, Wang D. Lactic acid bacteria protects Caenorhabditis elegans from toxicity of graphene oxide by maintaining normal intestinal permeability under different genetic backgrounds. Sci Rep. 2015;5:17233. doi: 10.1038/srep17233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sun S, Mizuno Y, Komura T, Nishikawa Y, Kage-Nakadai E. Toll-like receptor homolog TOL-1 regulates Bifidobacterium infantis-elicited longevity and behavior in Caenorhabditis elegans. Biosci Microbiota Food Health. 2019;38:105–110. doi: 10.12938/bmfh.18-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Poupet C, Saraoui T, Veisseire P, Bonnet M, Dausset C, Gachinat M, Camarès O, Chassard C, Nivoliez A, Bornes S. Lactobacillus rhamnosus Lcr35 as an effective treatment for preventing Candida albicans infection in the invertebrate model Caenorhabditis elegans: First mechanistic insights. PLoS One. 2019;14:e0216184. doi: 10.1371/journal.pone.0216184. [DOI] [PMC free article] [PubMed] [Google Scholar]