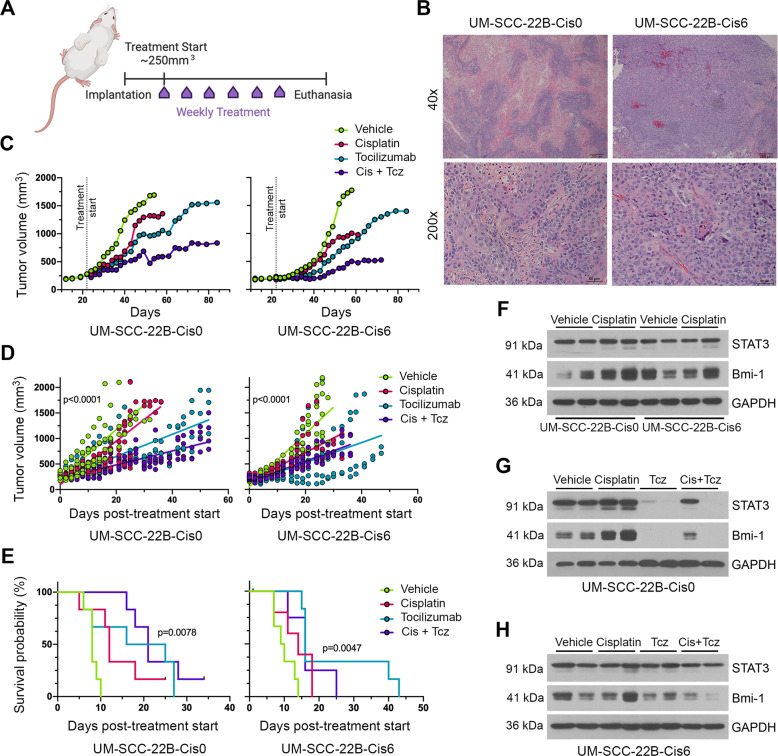
Fig. 6. Tocilizumab inhibits cancer stemness in a Cisplatin-resistant xenograft model of HNSCC.
A Schematic diagram of the study design. Xenograft tumors were generated with UM-SCC-22B-Cis0 (Cisplatin-naive) or UM-SCC-22BCis6 (Cisplatin-resistant) cells. Treatment began once tumors reached an average of 250 mm3. Mice received weekly injections of either vehicle, Cisplatin (5 mg/kg, i.p.) and/or Tocilizumab (10 mg/kg, i.p.) for up to 8 weeks. Mice were killed either at experiment endpoint (8 weeks post treatment start) or when reaching our cutoff tumor volume (2000 mm3). B Representative images of histological sections stained for H&E of xenograft tumors generated with UM-SCC-22B-Cis0 or UM-SCC-22BCis6 cells. Scale bars represent 200 µm at ×40 magnification and 50 µm at ×200 magnification. C Line graph depicting mean tumor volume over time after treatment with vehicle, Cisplatin, and/or Tocilizumab. Tumor measurements were taken three times per week until study endpoints. D Simple linear regression model of mean tumor volumes over the duration of the experiment. E Kaplan–Meier graph for survival, as defined by time to doubling of tumor volume, as compared to pretreatment tumor volume (n = 6 per experimental condition). F Western blottings for STAT3 and Bmi-1 expression in representative xenograft tumor tissue lysates (UM-SCC-22B-Cis0 or UM-SCC-22BCis6 xenograft models) comparing vehicle-treated with Cisplatin-treated mice. G Western blottings for STAT3 and Bmi-1 in representative UM-SCC-22B-Cis0 (Cisplatin-naive) tumor lysates collected from mice treated with vehicle, Tocilizumab, and/or Cisplatin. H Western blottings for STAT3 and Bmi-1 in representative UM-SCC-22BCis6 (Cisplatin-resistant) tumor lysates collected from mice treated with vehicle, Tocilizumab, and/or Cisplatin.