Figure 2:
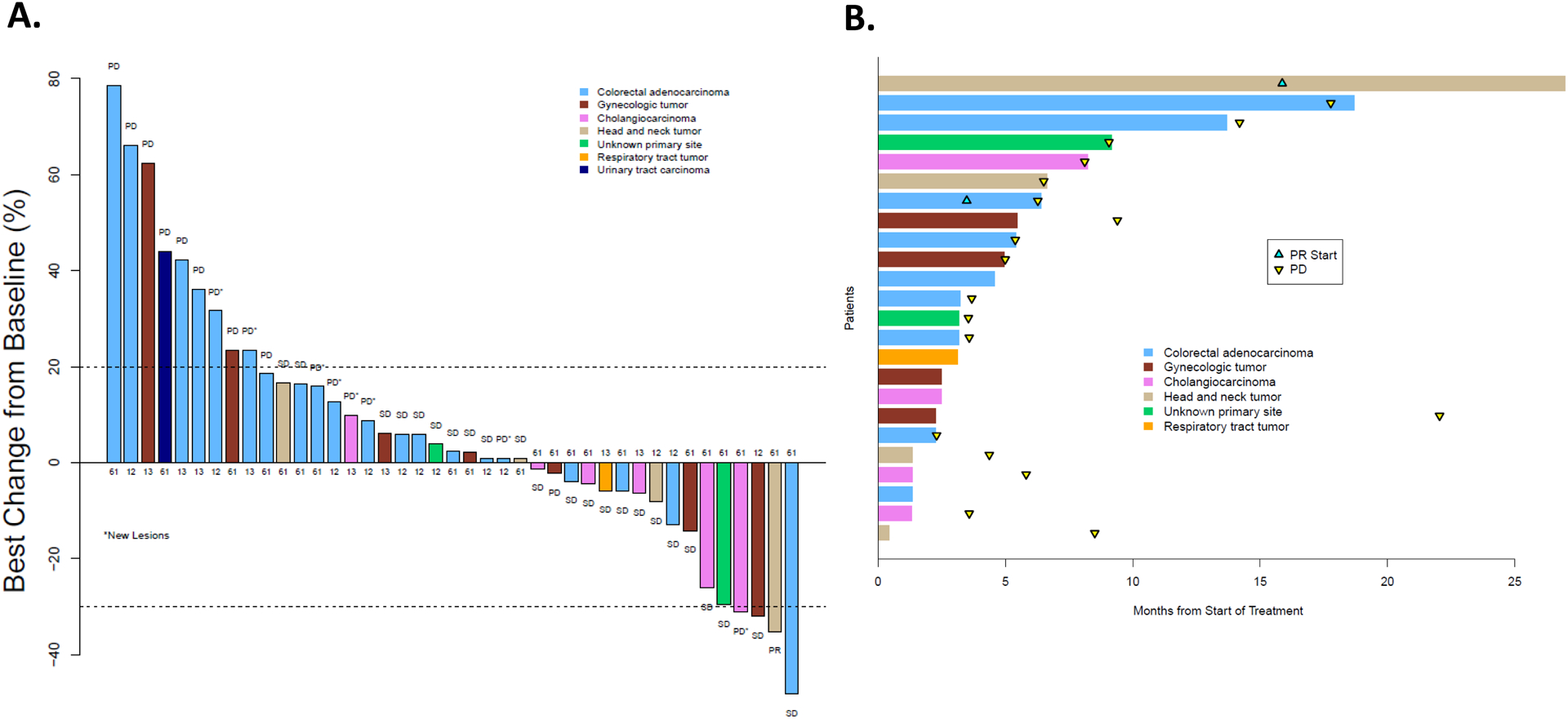
(A) Best overall response according to RECIST criteria in the 41 evaluable patients who remained on this subprotocol. The number associated with each tumor designates the NRAS codon that was mutated. (B) treatment duration of the 24 patients whose best response was stable disease or a partial response. Abbreviations: Progressive disease (PD), stable disease (SD), and PR (partial response).
