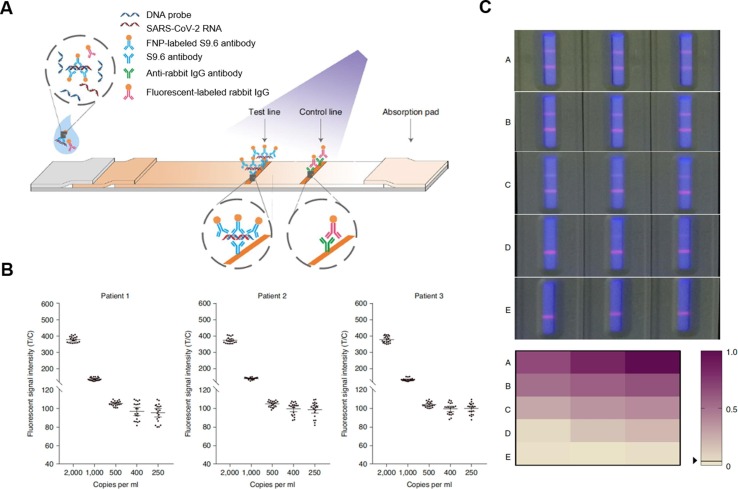
Fig. 3.
Design of a lateral flow immunoassay (LFIA) based on S9.6 antibody-labelled europium-chelate fluorescent nanoparticles (S9.6-FNPs) to detect SARS-CoV-2 RNA. (A) Schematic illustration of the working principles of the S9.6-FNPs-based LFIA. (B) The fluorescent readouts of the assay when testing serially diluted throat swab samples from three SARS-CoV-2-positive patients. The fluorescent readouts are presented as the ratio of fluorescent intensities at the test line to those at the control line (T/C). The viral RNA loads in the samples were measured using polymerase chain reaction (PCR) and expressed in copies ml−1. The detection limit of the assay for viral RNA was determined to be 500 copies ml−1 with a positive rate higher than 95% (n = 20, means ± the standard deviation). (C) Photographs of typical assay results obtained from clinical samples under a fluorescent light source. The results were grouped into A, B, C, D, and E based on their fluorescent readouts. The fluorescent readouts decreased from group A to E. Group E consisted of SARS-CoV-2 negative results entirely. The fluorescent readouts were normalized to the maximum readout on a scale from 0 to 1. The normalized fluorescent readouts were presented in a color-gradient matrix. Reproduced with permission from ref [17]. Copyright 2020, Spring Nature.