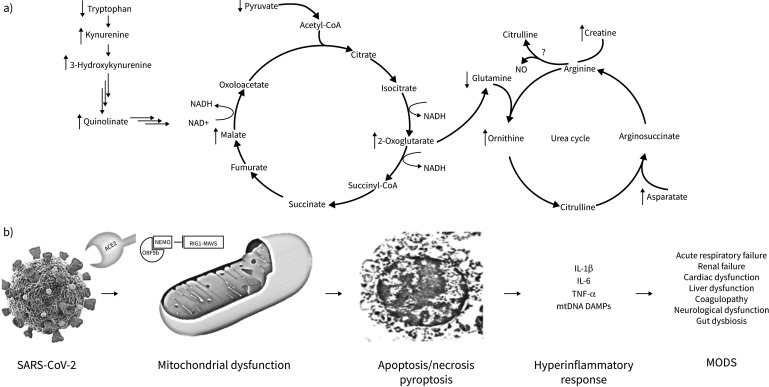
FIGURE 1.
Metabolomics changes due to SAR-CoV-2 are predictors of patient acute and long-term outcomes and reflect a bioenergetic crisis likely due to viral hijacking of the mitochondria. a) Metabolomics changes found due to SARS-CoV-2 infections commonly lead to disruption of the kynurenine pathway, TCA cycle and the urea cycle. It is unknown if arginine is converted to citrulline and NO; however, due to the increase in ornithine and creatine, Jia et al. [10] speculate that the urea cycle metabolises arginine instead of the NO producing pathway. b) SARS-CoV-2 is internalised into the cell via the angiotensin converting enzyme 2 (ACE2) receptor. Many viral proteins, including ORF7a and ORF9b locate within the mitochondria. These proteins can inhibit RIG1-MAVS (retinoic acid-inducible gene I-mitochondrial antiviral signalling protein)-dependent interferon signalling, enhance viral replication and disrupt mitochondrial function. This can ultimately lead to cell death via apoptosis, necrosis or pyroptosis, the release of proinflammatory cytokines as well as mitochondrial DAMPs (damage-associated molecular patterns) and ultimately cause multiple organ dysfunction syndrome (MODS). Jia et al. [10]also demonstrate that many of the predictive metabolites altered due to SARS-CoV-2 strongly correlate with proinflammatory cytokines. TNF: tumour necrosis factor; NEMO: nuclear factor κB essential modulator.