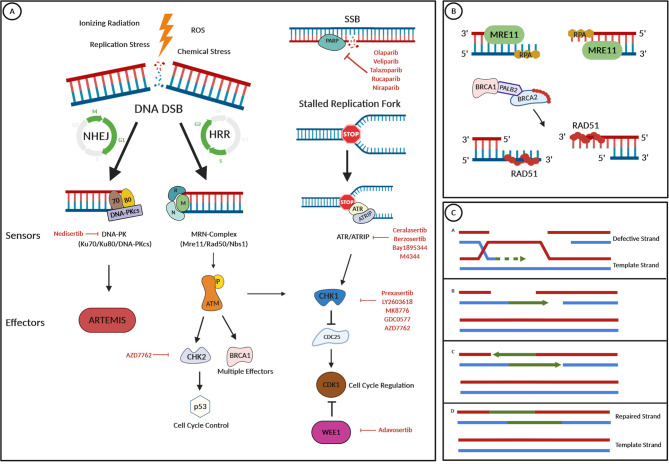
Figure 1.
DNA damage repair pathways and drug targets. (A) Several internal and external stressors such as ionizing radiation, replication stress, ROS, or chemical stress can lead to DNA double-strand breaks (DNA DSBs). Depending on cell cycle progression and the availability of a template strand, either the nonhomologous end joining (NHEJ) or the homologous recombination repair (HRR) pathway are the mechanism of choice for DNA repair. In the NHEJ pathway, DNA damage is recognized by the “DNA-dependent protein kinase” (DNA-PK), a nuclear serine/threonine protein kinase complex, composed of a large catalytic subunit named DNA-PKcs and a heterodimer of Ku proteins (Ku70/80). Then, the nuclease Artemis is activated and cleaves 5′- and 3′-DNA overhangs. In HRR, which only occurs in the S and the G2 phase of the cell cycle, a protein complex termed MRN (which is composed of MRE11, Rad50, and Nbs1) recognizes DNA DSBs. After its activation by phosphorylation, the ATM kinase works as a master effector protein for HRR by activating several downstream effectors such as the CHK1 or the CHK2 kinase or the BRCA1 protein, having effects on cell cycle progression via p53 control or CDK1 inhibition. Stalled replication forks lead to an arrest of DNA replication and such replication stress could lead to DNA DSBs. To prevent this and to stop cell cycle progression, the ATR protein kinase is recruited and binds to its partner protein ATRIP. Cell cycle regulation and repair mechanisms are then controlled via CHK1 activation. Repair of DNA single-strand breaks (SSBs) is initiated by PARP enzymes. (B) In HRR, the MRE protein acts as a nuclease and resects the broken DNA ends, which results in the overhanging of single-stranded 3′-ends. Protection from further resection or modification of ssDNA is reached by the binding of the RPA protein. RAD51 is essential for HRR, as it facilitates DNA unwinding, stretching, and invasion into the template strand. To prevent its early polymerization on DNA, RAD51 proteins are kept inactive by binding to BRCA2, which is recruited to the broken DNA in a complex with BRCA1 and the linker protein PALB2 after detection of DNA damage. The RAD51-DNA filament is then capable of invading the template strand. (C) The RAD51-DNA filament invades the template strand and searches for extended homologous regions, which are then stabilized by base pairing. RAD51 disassembles and leaves a heteroduplex of the defective DNA strand and the template strand. The invading strand is extended by a DNA polymerase, and after elongation, the newly synthesized strand segment is displaced and finally ligated with its original strand endings. This figure was created using the BioRender.com online tool.