Figure 1.
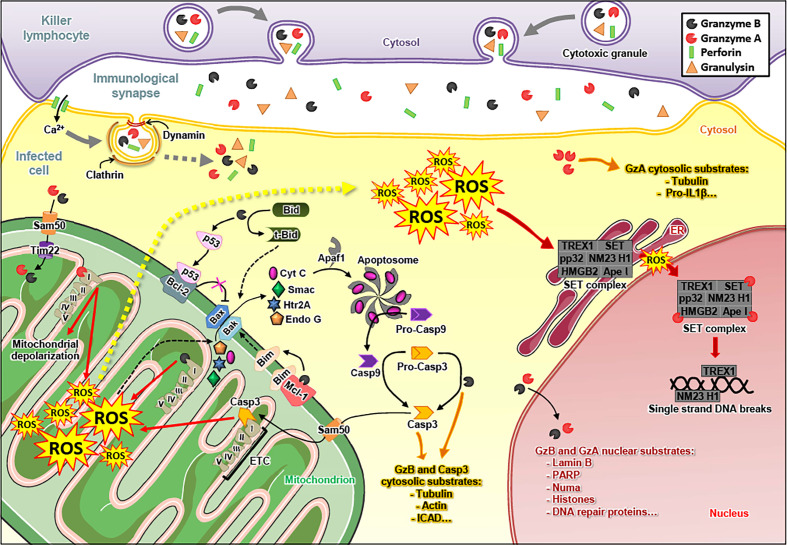
Granzyme A and Granzyme B induce apoptosis of infected cells. The killer lymphocyte releases the content of its granules in the immunological synapse, i.e. the immune effectors granzyme A (GzmA), granzyme B (GzmB), perforin (PFN) and granulysin (GNLY). The endocytosis of GzmA, GzmB, PFN and GNLY in the infected cell is mediated by a PFN-dependent calcium (Ca2+) influx and relies on clathrin and dynamin. Once in the cytosol, GzmA and GzmB cleave various substrates. GzmB triggers the formation of Bax/Bak pores in the outer mitochondrial membrane by cleaving Bid (in truncated-Bid, t-Bid), Mcl-1 (which releases Bim) (12) and p53 (which inhibits Bcl2) (13). GzmB also directly cleaves and matures pro-caspase 3 (Pro-Casp3) into active caspase 3 (Casp3). GzmB and caspase 3 target similar substrates that induce apoptosis of the infected cell. GzmA and GzmB enter the mitochondria – via Sam50 and Tim22 – where they target subunits of the electron transport chain (ETC) complex I, leading to the production of reactive oxygen species (ROS). The ROS favor the release of apoptogenic factors through the Bax/Bak pores, such as cytochrome C (Cyt C), Smac, Htr2A and endonuclease G (Endo G) in the cytosol. Cyt C binds Apaf1 to form the apoptosome, which matures the pro-caspase 9 (pro-Casp9) into active caspase 9 (Casp9). It is noteworthy that caspase 3, either activated by GzmB or caspase 9, also reaches the mitochondria – via Sam50 – where it cleaves a subunit of ETC complex I, leading to the production of ROS. Following these events, ROS concentration increases in the cytosol of the infected cell. The cytosolic ROS are involved in the translocation of the SET complex from the endoplasmic reticulum (ER) to the nucleus, where it is cleaved by GzmA and turns into a DNA degrading complex. GzmA and GzmB also reach the nucleus where they cleave nuclear substrates, such as lamin B, histones and PARP-1.