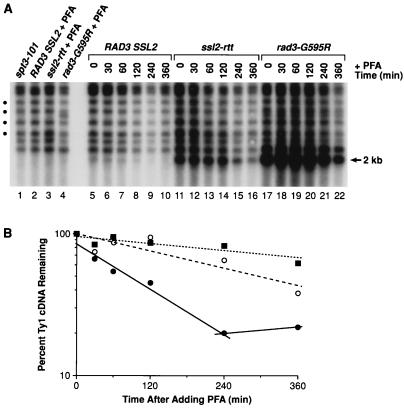
FIG. 6.
Stability of Ty1 cDNA in ssl2-rtt and rad3-G595R mutants. (A) Southern blot analysis of Ty1 cDNA from isogenic strains that were treated with the RT inhibitor PFA. DNA was prepared from strain DG789 (spt3-101) (lane 1), which was not treated with PFA, and strains GRF167 (RAD3 SSL2 wild type) (lane 2), DG1722 (ssl2-rtt) (lane 3), and BLY157 (rad3-G595R) (lane 4), which were grown in the presence of PFA (200 μg/ml) for about 48 h. The resulting DNA samples were digested with PvuII and analyzed by Southern blot hybridization with a 32P-labeled probe from the Ty1 RT coding region (40). Decay rates of Ty1 cDNA were determined for the RAD3 SSL2 wild-type strain (lanes 5 to 10), the ssl2-rtt mutant (lanes 11 to 16), and the rad3-G595R mutant (lanes 17 to 22), which were treated with PFA for various periods of time and then processed for Southern blot analysis described as in Materials and Methods and in the legend to Fig. 5. An aliquot of cells was withdrawn from each culture at the time of PFA addition (0 min) (lanes 5, 11, and 17) and at 30 min (lanes 6, 12, and 18), 60 min (lanes 7, 13, and 19), 120 min (lanes 8, 14, and 20), 240 min (lanes 9, 15, and 21), and 360 min (lanes 10, 16, and 22) after PFA addition. The level of unincorporated Ty1 cDNA in each culture was monitored by determining the amount of a 2.0-kb fragment generated by PvuII digestion. Four conserved Ty1-chromosomal DNA junction fragments (circles) were used for normalization of the Ty1 cDNA level. (B) Ty1 cDNA decay curves were plotted on a log scale as the percentage of Ty1 cDNA remaining relative to the level of Ty1 cDNA at time zero versus elapsed time. All Ty1 cDNA hybridization signals were normalized to that of the four conserved Ty1-chromosomal DNA junction fragments shown in panel A. The kinetics of Ty1 cDNA decay for the wild-type (closed circles, solid line), ssl2-rtt (open circles, dashed line), and rad3-G595R (closed squares, dotted line) strains are shown.