Abstract
Cardiac amyloidosis (CA) is an infiltrative cardiomyopathy caused by the extracellular deposition of amyloid fibrils in the myocardium. Although cardiac amyloidosis patients primarily present with heart failure symptoms, arrhythmias and conduction system disease are frequently encountered. Atrial fibrillation (AF) is observed in up to 70% of patients at the time of diagnosis, and patients typically have controlled ventricular rates caused by concomitant conduction system disease. Thromboembolic risk is particularly high in patients with CA and AF, and left atrial thrombi have been observed even in the absence of clinically diagnosed AF. Atrioventricular nodal and infra-Hisian disease are common, and permanent pacemakers are frequently required. The use of implantable cardioverter-defibrillators in this population is controversial. This review summarizes the published data and therapeutic strategies surrounding arrhythmias and conduction system disease with the goal of aiding clinicians managing the clinical complexities of CA.
Key Words: atrial fibrillation, cardiac amyloidosis, cardiac implantable electronic devices, conduction abnormalities, congestive heart failure
Abbreviations and Acronyms: AF, atrial fibrillation; AL, light chain; ATTR, transthyretin; AV, atrioventricular; CA, cardiac amyloidosis; DCCV, direct current cardioversion; ICD, implantable cardioverter-defibrillator
Central Illustration
Highlights
-
•
Cardiac arrhythmias and conduction system disease are common in CA.
-
•
Management of AF is complicated by difficulty in maintaining sinus rhythm and thromboembolic complications.
-
•
Atrioventricular nodal and infra-Hisian disease are common, and the optimal choice of device is controversial.
-
•
Prospective studies are required to establish optimal management strategies for arrhythmias and conduction disease.
Cardiac amyloidosis (CA) is an increasingly recognized infiltrative cardiomyopathy caused by the extracellular deposition of insoluble protein fibrils (1). Two major subtypes of CA exist: light chain (AL) and transthyretin (ATTR) CA (2). Whereas AL-CA results from the excessive production of antibody light chains that misfold and deposit in tissues, ATTR-CA is caused by the extracellular deposition of misfolded monomers of transthyretin (2). ATTR-CA is classified by the sequence of the TTR gene as either wild-type (ATTRwt-CA), in which no sequence variant is identified, or hereditary/variant ATTR-CA (ATTRv-CA), in which a sequence variant is present (2).
In recent years, there has been a steady increase in reported CA (3). This is due, in part, to the introduction of noninvasive diagnostic modalities, such as strain echocardiography and scintigraphic nuclear imaging (4), in addition to advancements in treatment options (5). Recent studies have suggested that ATTRwt-CA is present in 13%-17% of patients with heart failure with preserved ejection fraction (HFpEF) and an increased left ventricular wall thickness (6,7), and up to 1 in 7 of those with severe symptomatic aortic stenosis (AS) undergoing transcatheter valve replacement (8).
Clinically, CA can present in a variety of manners depending on disease subtype and stage. Although patients typically present with signs and symptoms of heart failure, a somewhat underappreciated manifestation common to all forms of CA is conduction system disease. Although atrial arrhythmias, particularly atrial fibrillation (AF), are well-documented in this condition, there are less data in the published data pertaining to ventricular arrhythmias and atrioventricular (AV) nodal disease. In this paper, we review the pathogenesis, diagnosis, and management of atrial arrhythmias and conduction system disease in CA.
Pathogenesis of Conduction Disease
The pathogenesis of conduction system disease in CA is multifactorial. First, amyloid deposition results in thickening and disarrangement of the otherwise organized myocardial architecture. This, in turn, disrupts the transmission of electrical impulses along conduction fibers (2). Second, cytotoxicity may play a role, because certain forms of amyloid precursor proteins are well recognized noxious agents that induce apoptosis through oxidative stress (9). For instance, infusion of light chains from patients with AL-CA into isolated mouse hearts has been shown to induce diastolic dysfunction independent of amyloid deposition in vitro (9).
Transthyretin is a well-known neurotoxic agent. Its noxious effects have been proven both in vitro (10,11), and in vivo (12). Data on cardiac myocyte toxicity are only emerging. In vitro studies demonstrate that numerous ATTR variants are cytotoxic to human myocardial cell lines in a concentration-dependent manner (13). Furthermore, the mechanism of cytotoxicity not only involves oxidative stress and apoptosis, but also dysregulation of intracellular calcium signaling causing action potential prolongation.
Early cardiac sympathetic denervation may also contribute to arrhythmogenesis. Recent nuclear imaging studies, using 123iodine-metaiodobenzylguanidine (123I-MIBG) scintigraphy as a marker of cardiac sympathetic activity, have found that denervation occurs before detectable amyloid deposition via bone scintigraphy (14). In ATTRv-CA, reduced myocardial MIBG uptake is a marker of disease severity and is associated with poor prognosis (15). Although causality has yet to be proven, neurotoxic amyloid deposition within the interstitial space with consequent interstitial remodeling is thought to drive loss of sympathetic nerve fibers in CA (16).
Given the unique position of the cardiac conduction system at the intersection between myocardial and neuronal tissue, and the ability of ATTR to disrupt cytoplasmic calcium signaling, the potential molecular mechanisms underlying the cytotoxicity of transthyretin on this specialized system are evident (Central Illustration). Although little evidence currently exists on the toxicity of amyloid on these uniquely conducting myocytes, its clinical sequalae are abundantly clear through the prevalence of rhythm disturbances among patients with CA.
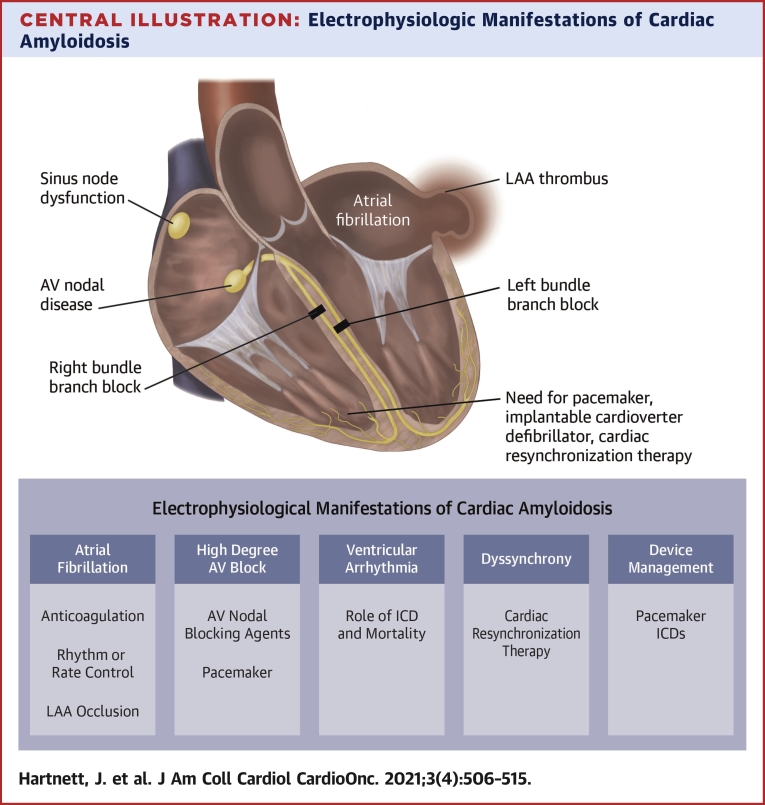
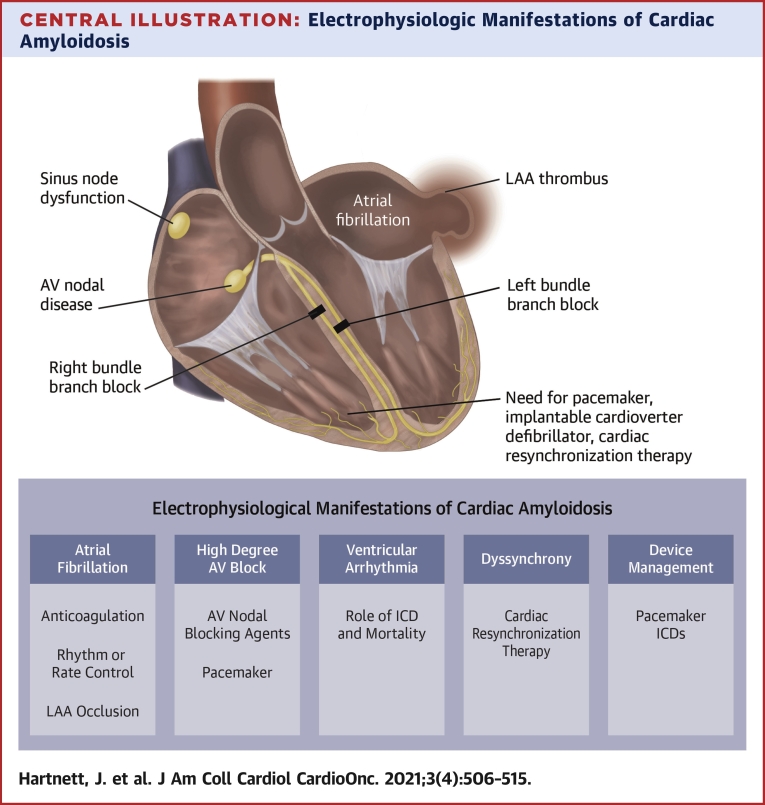
Central Illustration.
Electrophysiologic Manifestations of Cardiac Amyloidosis
Electrophysiological manifestations (dark blue box) and management considerations (light blue box) of cardiac amyloidosis include atrial fibrillation, LAA thrombus, and atrioventricular nodal disease. There is also a risk of left and right bundle branch block in both light chain and transthyretin cardiac amyloidosis. ICD = implantable cardioverter-defibrillator; LAA = left atrial appendage.
Atrial Fibrillation
Atrial fibrillation is the most common rhythm disturbance in CA. The high prevalence of AF in CA is not difficult to envisage, given the predisposition for amyloid fibrils to deposit in the atria in addition to the chronically elevated intra-atrial pressures, resulting in left atrial dilation.
Evidence from prospective and retrospective studies have suggested that the prevalence of AF in CA is between 10% and 69%. In one of the earliest large-scale longitudinal studies, Rapezzi et al (17) catalogued the rhythm disturbances evident in patients with CA across 2 major Italian centers. This study included 233 patients, of whom 157 had AL-CA, 61 had ATTRv-CA, and 15 were diagnosed with ATTRwt-CA. Although no clear pattern of conduction abnormalities was evident, the most pertinent finding was that a normal ECG in CA is rare, evident in only 5% of those with AL-CA, 10% in ATTRv-CA, and 0% in ATTRwt-CA. Although very few patients with ATTRv-CA were included (n = 15), AF was considerably more frequent in the ATTRwt-CA cohort (27% vs 12%; P = 0.046), likely caused by the older age of those with ATTRwt-CA (17).
This finding is in keeping with more recent studies across Europe and the United States. In an analysis of 102 biopsy-proven cases of ATTRwt-CA, Pinney et al (18) reported that 38% of patients with ATTRwt-CA were in AF at baseline, compared with only 11% of patients with AL-CA. Similarly, another retrospective study comprising 360 patients from the Mayo Clinic noted a prevalence of AF and atrial flutter of 62% among individuals with ATTRwt-CA (3). A further single-center retrospective study from the Cleveland Clinic including 382 patients with ATTR-CA found that AF was present in 76% of patients with ATTRwt-CA and 53% of those with ATTRv-CA (19).
Compared with age-adjusted rates in the general population, intracardiac thrombi and thromboembolic events are far more common in those with CA. An autopsy series of 116 patients with CA reported intracardiac thrombus in 33% of patients, of whom 40% had multiple thrombi (Figure 1) (20). Factors associated with the development of thromboembolism included AL-CA and left ventricular diastolic dysfunction (20). Although autopsy studies may overestimate the prevalence of clinically significant thrombi, the same group reported similar findings using echocardiography in a follow-up study (21). Again, left ventricular diastolic dysfunction, AL-CA, and AF were independently associated with thrombus formation (21). Strikingly, this study highlighted the degree of thrombogenicity in AL-CA: 25% of patients with AL-CA without a history of AF had intracardiac thrombi on imaging, whereas no patient with ATTR-CA without a history of AF had intracardiac thrombus (21). In a study of 100 consecutive patients with ATTR-CA undergoing transesophageal echocardiography before direct current cardioversion (DCCV), Donnellan et al (22) found that the risk of left atrial thrombus in CA is independent of CHA2DS2-VASc score. Given the high rates of atrial myopathy and thrombi, anticoagulation should be prescribed to all patients with CA and AF in the absence of a prohibitively high bleeding risk, regardless of CHA2DS2-VASc score (2). In addition to the higher rates of intracardiac thrombus, patients with CA and AF are also at increased risk of cardiogenic shock compared with those with CA without AF (23). This finding is most likely caused by the loss of AV synchrony and of the atrial contribution to diastolic left ventricular filling. Despite these increased rates of complications, however, no studies to date have demonstrated an association between AF and increased mortality.
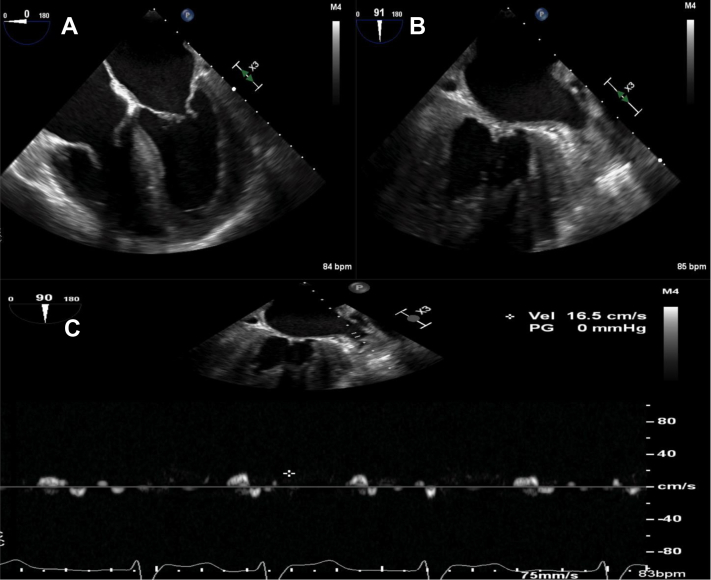
Figure 1.
Echocardiographic Features of Atrial Myopathy in Cardiac Amyloidosis
Transesophageal echocardiogram in a patient with transthyretin cardiac amyloidosis demonstrating the following: (A) an enlarged left atrium; (B) left atrial appendage thrombus with overlying sludge; and (C) low left atrial appendage emptying velocity (16.5 cm/s).
Management of AF
Despite its prevalence, there is a paucity of high-quality data on optimal therapeutic strategies for AF in CA. Given both the unique pathophysiology and increased risk associated with AF in CA, it is unsurprising that the intricacies of management are also different to that of AF more broadly.
This is no truer than in the realm of medical management. Rate control strategies are poorly tolerated in CA, because they may result in a low-output state and hemodynamic deterioration. Due to the restrictive physiology in CA, stroke volumes are relatively fixed and maintenance of cardiac output is intimately dependent on heart rate and left ventricular diastolic filling. Consequently, patients with CA are poorly tolerant of negative ionotropic agents, such as beta-blockers and calcium channel blockers, which reduce cardiac output, and high heart rates, which decrease diastolic filling time (24, 25, 26). Interestingly, toxicity may be precipitated by an ability of amyloid fibrils to bind pharmacotherapies and thus enhanced concentration of the drug available to receptors within the myocardium. Case reports of short-term left ventricular failure secondary to these agents have been published, and this binding capacity has been proven for digoxin in early in vitro studies (26). As previously noted, often times AV nodal conduction disease is present in these patients, allowing for rate-controlled AF without medications. Nevertheless, rate-control agents are sometimes necessary for patients with AF and CA (19,27). Given digoxin’s potential deleterious effects, it was an absolute contraindication in CA patients. However, some centers are now using it cautiously in select patients.
Antiarrhythmic therapy (AAT) is less commonly prescribed in CA, and there is currently a paucity of studies investigating the efficacy and safety of AAT. Given the preponderance of coexisting heart failure and renal impairment, many AAT agents commonly employed clinically are contraindicated in patients with CA. For instance, Class IC agents such as flecainide and propafenone are contraindicated in patients with structural heart disease, including left ventricular hypertrophy (28). Similarly, certain Class III agents, such as dronedarone and sotalol, are contraindicated in patients with significant renal impairment and heart failure (29). Consequently, amiodarone and dofetilide are the most widely utilized agents (19). Given its predominant renal route of excretion, both renal function and corrected QT interval need to be closely monitored in patients with CA initiated on dofetilide, and dofetilide is contraindicated in patients with a creatinine clearance <40 mL/min (29). Our group previously found that AAT was more effective when employed earlier in the disease course, and that maintaining normal sinus rhythm appeared to confer a mortality benefit (19). With respect to DCCV, previous studies have found that both procedure cancellations and periprocedural complications are far more common in those with CA compared with age- and sex-matched control subjects (30,31). In a review of DCCV outcomes among 58 patients with CA and AF at the Mayo Clinic, pre-DCCV intracardiac thrombi were detected in 28% of those with CA compared with 2.5% in the control group (P < 0.001) (30). Procedural complications, including stroke and severe postconversion bradyarrhythmias, were also more common in those with CA (14% vs 2%; P = 0.008) (30). In a similar manner to AAT, our group previously reported that DCCV was substantially more effective when performed earlier in the course of CA. Of 119 patients with ATTR-CA who underwent DCCV, only 33% of patients with stage 3 ATTR-CA remained in sinus rhythm at 30 days following DCCV, compared with 90% of patients with stage 1 disease (P < 0.001) (19). Extensive low-voltage areas were observed on electroanatomic mapping in patients with ATTR-CA (Figure 2).
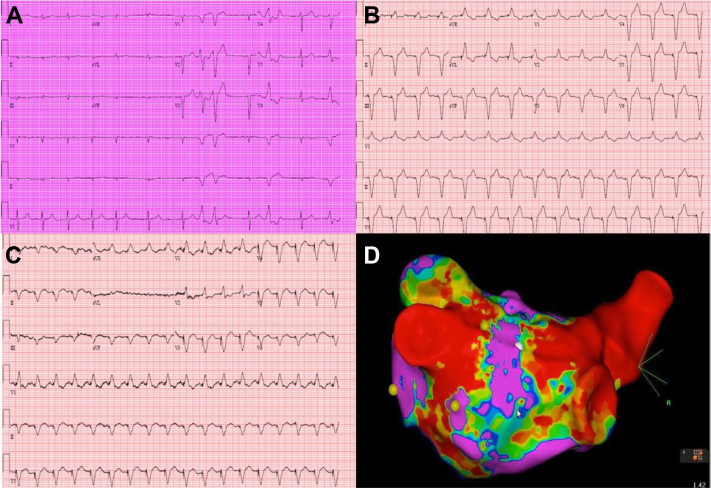
Figure 2.
Electrocardiographic Features of Cardiac Amyloidosis
Electrocardiograms demonstrating (A) atrial fibrillation, (B) right ventricular apical pacing, and (C) biventricular pacing in patients with transthyretin cardiac amyloidosis. (D) An electroanatomic map in the posteroanterior projection showing extensive atrial scarring (red) in an enlarged left atrium. The areas in purple denote healthy left atrial myocardium.
Despite the ubiquity of anticoagulation in this population, there are very little data comparing anticoagulants. Early studies suggest that warfarin is more commonly used, prescribed in up to 79% of patients in 1 publication (27). However, a more recent retrospective analysis of 217 patients noted that 53.5% of patients were treated with direct oral anticoagulants, in comparison to only 35.9% on warfarin (32). Notably, no difference in the rate of thromboembolic events was found between warfarin and direct oral anticoagulant therapy (32). The role of left atrial appendage (LAA) occlusion devices in patients with CA is currently controversial. Although a diagnosis of CA was not an exclusion criterion in the prospective, randomized clinical trials that demonstrated the noninferiority of LAA closure relative to warfarin (33,34), some have made the argument that a foreign body may serve as a nidus for thrombus formation in the enlarged, dysfunctional atria frequently observed in individuals with CA. The role of LAA closure in CA is currently an active area of research.
Catheter ablation for AF in CA has been studied in 4 small observational studies (35, 36, 37, 38). In all 4 studies, the procedure was generally safe with few perioperative complications. Notably, the rate of periprocedural thromboembolic events was similar in CA and non-CA patients. However, arrhythmia recurrence is common following ablation. Our group studied 24 patients with ATTR-CA undergoing AF ablation and found that 58% developed recurrent arrhythmia during a mean follow-up of 39 months (39). Rates of recurrence were high in those with more advanced ATTR-CA, with 90% of those with stage 3 disease experiencing recurrence, compared with 36% of those with stage 1 ATTR-CA (P < 0.0001). Ablation was associated with substantially lower rates of hospitalization (18% vs 72%; P < 0.0001) and mortality (29% vs 75%; P = 0.01) (39). Furthermore, in a study of 26 patients with CA and atrial arrhythmias, Tan et al. (36) noted improvements in New York Heart Association (NYHA) functional class symptoms 6 months post ablation.
Ultimately, conclusions differ. Authors of earlier studies suggest the role of ablation in CA-related AF is limited given the high recurrence (35). More extensive ablation beyond the pulmonary veins is required, because amyloid deposition occurs diffusely in CA and the pulmonary veins are not the only source of AF. In our experience, if ablation is to be considered, it should be performed early in the disease course.
Sinus Node Dysfunction
Sinus node dysfunction (SND) is defined as an inability of the sinoatrial node to generate a heart rate that meets physiological needs. It can manifest in a variety of ways, including sinus bradycardia, sinus pauses, or sinus arrest. Despite the high prevalence of conduction disease in CA and the predisposition for amyloid to deposit in the atria, SND in CA is not well studied. Most studies investigating conduction system disease in CA do not discuss SND. Available data suggest it is uncommon. In a recent single-institution retrospective study of 369 patients followed over 28 months, SND occurred in 7% of patients (40). It was more common in ATTRv-CA than in ATTRwt-CA (8% vs 6%); however, statistical significance was not reached (40). Although infiltrative conditions, including CA, have been considered classical causes of sinoatrial node pathology, published outcomes in CA are lacking.
AV Nodal Disease
AV conduction disease is common in CA. In a single-center retrospective study including 369 patients with ATTR-CA, 9.5% had pacemakers implanted for high-grade AV block before their diagnosis with CA (40). During a follow-up period of 28 months, a further 11% developed a pacemaker requirement for high-grade AV block (40). First-degree AV block was very common in this population, occurring in 49% of those with ATTRwt-CA and 43% with ATTRv-CA (40). No significant differences in the development of AV block were observed across disease stages, and only a QRS duration ≥120 milliseconds was associated with an elevated risk of AV block (40).
In an Italian study of 233 patients, Rapezzi et al (17) found that first-degree AV block was present in 18% of those with AL-CA, but up to 33% of those with ATTRwt-CA. Moreover, 13% of patients with ATTRwt-CA had pacemakers implanted prior to diagnosis compared with 3% of those with ATTRv-CA (17). Meanwhile, Pinney et al (18) noted rates of first-degree AV block of 11 and 15%, respectively, in those with ATTRwt-CA and AL-CA.
Pacemakers are commonly required in patients with CA. Analysis of 145,900 hospitalizations across the United States demonstrated that 3.9% of those with CA and documented arrhythmias had pacemakers (23). One study observed that 10% of patients with ATTRwt-CA and 7% with ATTRv-CA had pacemakers in situ at the time of CA diagnosis (40). In a 10-year retrospective review of 262 patients with ATTRv, a pacemaker was inserted in 45% of cases (41). Compared with patients with HFpEF without a diagnosis of CA, patients with CA and HFpEF require pacemakers far more commonly (43.8% vs 11.5%; P = 0.004) (6). Most reports agree that pacemakers are most common in ATTRwt-CA, followed by ATTRv-CA and, finally, AL-CA. However, advanced age at the time of diagnosis could confound these results.
Progression of conduction system disease is common and often leads to increased right ventricular (RV) pacing burden with time. Authors from Duke University examined the longitudinal electrophysiology data from pacemaker interrogation in 34 patients with CA and cardiac implantable electronic devices. Pronounced dependence on RV pacing was evident over time, from an average pacing burden of 35.5% at 6 months postinsertion up to 96.2% 5 years later (42).
Furthermore, a high burden of RV pacing is associated with deleterious consequences. In a retrospective observational cohort study of 78 patients with ATTR-CA and cardiac implantable electronic devices, a pacing burden >40% was shown to result in adverse structural and clinical consequences, including worsening NYHA functional class, left ventricular ejection fraction (LVEF), and an increased severity of mitral regurgitation (43). Patients with CA appear to be particularly vulnerable to the interventricular and intraventricular dyssynchrony brought about by RV pacing, given their restrictive physiology. As a corollary, biventricular pacing has been associated with improvements in NYHA functional class, LVEF, and mitral regurgitation severity (43). Summarizing this evidence, biventricular pacing should be considered when an indication for pacing emerges, because single-chamber pacing can result in a high RV pacing burden and eventual clinical deterioration.
Ventricular Arrhythmias
Data pertaining to ventricular arrhythmias (VAs) in CA are limited to several small retrospective studies, and the results have been somewhat conflicting. Although early publications observed high rates of VAs in AL-CA, more contemporary publications have reported much lower rates of sustained VAs, particularly among those with ATTR-CA (44,45).
There are numerous potential mechanisms driving ventricular arrhythmogenicity in CA. One proposal involves patchy amyloid fibril infiltration within the ventricular myocardium along with microvascular ischemia leading to the development of anatomical re-entrant circuits suitable for VA (46). Other mechanisms include preferential amyloid cytotoxicity to ventricular myocardial cells. This may explain the apparent higher prevalence of VAs in AL-CA over ATTR-CA.
Although VAs are common in CA, their effect on mortality may not be as significant as expected. In a study of AL-CA patients with presyncope or syncope who had a loop recorder implanted, bradycardia followed by PEA was the terminal rhythm in 62% of deaths, whereas only 1 episode of nonsustained ventricular tachycardia was evident from 272 loop recordings (47). Bradycardia and complete AV block were common prior to PEA—suggesting prophylactic pacemaker rather than implantable cardioverter-defibrillator (ICD) insertion could offer a survival benefit in patients with severe AL-CA. In a study of 19 patients with histologically proven AL-CA who underwent prophylactic ICD placement, Kristen et al (48) identified a low-voltage pattern on ECG, multiple ventricular ectopic beats, increased left ventricular wall thickness, and higher N-terminal pro–B-type natriuretic peptide levels as risk factors for PEA death. Anecdotally, vagal events can occur in patients with CA, particularly in those with AL-CA and autonomic neuropathy, which may lead to syncope and even asystole and death.
The role of ICDs in CA is contested and a survival benefit has not been proven (2). Although many patients with CA meet primary and secondary prevention criteria for ICD insertion according to consensus guidelines, there are many reasons why ICDs would be of limited value in this population. First, survival is often <1 year, which is generally a contraindication to ICD insertion. Second, SCD in these patients is often caused by PEA or asystole rather than VAs. Furthermore, high defibrillation thresholds caused by amyloid infiltration within the myocardial wall may make attempted ICD therapy unsuccessful.
Although these grounds appear reasonable for preclusion, the real-world data is considerably more complex. Numerous small, single-institution, retrospective studies have been published. Most of the early studies predominantly involve AL-CA patients, whereas more recent publications investigate ATTR-CA. The largest study used the U.S. National Cardiovascular Data Registry to select all registered CA patients following ICD insertion (n = 472) who were matched (1:5) with nonischemic cardiomyopathy control patients (49).
None thus far have demonstrated a convincing survival benefit to ICD insertion. Furthermore, 2 of the studies compared survival in CA patients with an inserted ICD vs a control population following ICD insertion. Compared with the control population, patients with CA have significantly reduced survival (49,50). Interestingly, the largest study found 1-year mortality for patients with CA and an ICD to be 26.9% vs 11.3% for nonischemic cardiomyopathy control patients (49).
However, hidden within the data is evidence of potential benefit in carefully selected patients. Throughout the studies, the rate of appropriate ICD therapy is high, up to 32% in the first year with subsequent rates of 9% per year thereafter (51). A higher rate of appropriate therapy in AL-CA over other forms was noted in multiple studies suggesting the risk of VAs may vary with amyloidosis subtype (44). In a retrospective analysis of 32 patients with CA who underwent ambulatory rhythm monitoring, Varr et al (52) reported a successful ICD therapy rate of 80%, leading to a survival of up to 19 months following ICD therapy. However, it must be stated that this survival is not compared to a nonamyloid control population. In a case-control study of 91 subjects with CA, Kim et al (50) report a median time to first therapy as low as 2.7 months in the CA group but significantly longer in the nonamyloid control group (2.7 months vs 29.39 months; P = 0.016).
These key findings, in conjunction with low rates of inappropriate ICD therapy (3%-4%) for supraventricular tachycardias (45,51) and low complication rates (3-8%) (48,51), hint at the conceivable efficacy of ICD in CA. It is likely ICD may be more efficacious in AL-CA over ATTR-CA. VAs are more common in the former, whereas SVTs are more common in the latter, resulting in higher rates of inappropriate ICD therapies (3 of 19 ATTR-CA patients in 1 small study) (44).
Recent publications have failed to identify any new positive associations of VA risk (45,51). One found that both cardiac biomarkers (troponin and N-terminal pro–B-type natriuretic peptide) and LVEF may be inversely correlated with VA risk (52). An explanatory hypothesis is that patients with CA and milder forms of HF are at higher risk because of the patchier amyloid infiltration evident at this disease stage. Furthermore, LVEF reduction only occurs in late-stage CA when mortality from nonarrhythmogenic etiologies is more common.
Consideration for ICD insertion will become an increasingly common scenario faced by clinicians treating patients with CA, because CA survival improves through new disease modifying therapies. The current data are conflicting and recommendations from major consensus guidelines are ambiguous. Further high-quality studies are necessary going forward to elucidate this potentially life-saving question. Consequently, shared decision making between the patient and the multidisciplinary team is critical when considering ICD insertion in CA.
Arrhythmias and Disease Modifying Therapies
A deeper understanding of the pathophysiology and biology governing amyloidosis has led to the development of numerous new disease-modifying therapies, particularly in ATTR-CA. Tafamidis is a transthyretin tetramer stabilizer that prevents dissolution into monomers and subsequent amyloid fibril formation. In a seminal Phase III randomized controlled trial published in 2018, tafamidis was shown to reduce both all-cause mortality and cardiovascular hospitalizations 30 months postrandomization compared with placebo in patients with ATTR-CA (5). Looking specifically at the effects of tafamidis in conduction disease, there have been no published data to date. Given its ability to prevent further fibril deposition, it is hoped that tafamidis therapy will slow electrophysiological deterioration if employed early in the disease course.
In ATTRv-CA, 2 new drugs have come to market. Patisiran and inotersen are disease-modifying oligonucleotide agents that slow production of transthyretin through RNA inhibition. Phase III trials have demonstrated their efficacy in improving symptomatic polyneuropathy in ATTRv amyloidosis (53,54). Despite being tested in patients with ATTRv amyloidosis with polyneuropathy, post hoc analyses have shown both to stabilize or reduce left ventricular wall thickness and improve cardiac output 1.5-2 years post–drug commencement (55). Their effects on conduction disease specifically are relatively unexplored. Patisiran had a lower rate of arrhythmias compared with placebo in the Phase III trial (30.6% vs 18.9%) (56). However, 2.7% of patients on the novel therapy develop AV nodal block requiring pacemaker insertion compared with 0% in the placebo group (56).
Arrhythmic considerations are not limited to therapies for ATTR-CA. For instance, carfilzomib, an irreversible proteasome inhibitor used in AL-CA, is associated with a 2-fold increase in risk of cardiovascular adverse events (57). In a meta-analysis of 2,594 patients receiving carfilzomib, cardiac arrhythmias occurred in 2.4% (57).
An abundance of potentially disease-modifying therapies are currently in the pharmaceutical pipeline, all with unknown cardiac sequelae. Because progressive cardiac disease is the most common cause of death in amyloidosis, greater emphasis on the cardiovascular effects of emerging therapies at the initial clinical trial stage is necessary. For the 3 market-approved therapies discussed, further post hoc analyses are required to delineate their suitability for and efficacy in CA associated arrhythmias.
Conclusions
Amyloid infiltration into the cardiac conduction system causes a plethora of electrophysiological dysfunction, most commonly atrial fibrillation. There is also increasing recognition of other important sequelae, including AV nodal disease and ventricular arrhythmias. The current evidence base for both electrophysiological interventions and the effect of new disease-modifying therapies on CA-related arrhythmias is sparse. However, in light of the ever-growing prominence of CA, these key clinical questions need to be clarified through high-quality collaborative prospective studies.
Funding Support and Author Disclosures
Dr Maurer has received grant support from National Institutes of Health R01HL139671, R21AG058348, and K24AG036778; has received consulting income from Pfizer, Eidos, Prothena, Akcea, GlaxoSmithKline, Intellia, Regeneron, and Alnylam; and his institution has received clinical trial funding from Pfizer, Prothena, Eidos, and Alnylam. Dr Hanna has served on advisory boards for Pfizer, Alnylam, Eidos, and Akcea. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Benson M.D., Buxbaum J.N., Eisenberg D.S. Amyloid nomenclature 2018: recommendations by the International Society of Amyloidosis (ISA) nomenclature committee. Amyloid. 2018;25:215–219. doi: 10.1080/13506129.2018.1549825. [DOI] [PubMed] [Google Scholar]
- 2.Ruberg F.L., Grogan M., Hanna M. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73:2872–2891. doi: 10.1016/j.jacc.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grogan M., Scott C.G., Kyle R.A. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68:1014–1020. doi: 10.1016/j.jacc.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 4.Gillmore J.D., Maurer M.S., Falk R.H. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 5.Maurer M.S., Schwartz J.H., Gundapaneni B. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 6.González-López E., Gallego-Delgado M., Guzzo-Merello G. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 7.Mohammed S.F., Mirzoyev S.A., Edwards W.D. Left ventricular amyloid deposition inpatientswith heart failure and preserved ejection fraction. J Am Coll Cardiol HF. 2014;2:113–122. doi: 10.1016/j.jchf.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castano A., Narotsky D.L., Hamid N. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao R., Jain M., Teller P. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation. 2001;104:1594–1597. [PubMed] [Google Scholar]
- 10.Mendes Sousa M.M., Cardoso I., Fernandes R. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am J Pathol. 2001;161:1935–1948. doi: 10.1016/s0002-9440(10)63050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sousa M.M., Yan S Du, Fernandas R. Familial amyloid polyneuropathy: receptor for advanced glycation end products-dependent triggering of neuronal inflammatory and apoptotic pathways. J Neurosci. 2001;21:7576–7586. doi: 10.1523/JNEUROSCI.21-19-07576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sousa M.M., Fernandes R., Palha J.A. Evidence for early cytotoxic aggregates in transgenic mice for human transthyretin Leu55Pro. Am J Pathol. 2002;161:1935–1948. doi: 10.1016/S0002-9440(10)64469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourgault S., Choi S., Buxbaum J.N. Mechanisms of transthyretin cardiomyocyte toxicity inhibition by resveratrol analogs. Biochem Biophys Res Commun. 2011;410:707–713. doi: 10.1016/j.bbrc.2011.04.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonker D.L., Hazenberg B.P.C., Nienhuis H.L.A. Imaging cardiac innervation in hereditary transthyretin (ATTRm) amyloidosis: a marker for neuropathy or cardiomyopathy in case of heart failure? J Nucl Cardiol. 2020;27:1774–1784. doi: 10.1007/s12350-018-01477-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Algalarrondo V., Antonini T., Théaudin M. Cardiac dysautonomia predicts long-term survival in hereditary transthyretin amyloidosis after liver transplantation. J Am Coll Cardiol Img. 2016;9:1432–1441. doi: 10.1016/j.jcmg.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Gimelli A., Aimo A., Vergaro G. Cardiac sympathetic denervation in wild-type transthyretin amyloidosis. Amyloid. 2020;27:237–243. doi: 10.1080/13506129.2020.1769059. [DOI] [PubMed] [Google Scholar]
- 17.Rapezzi C., Merlini G., Quarta C.C. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 18.Pinney J.H., Smith C.J., Taube J.B. Systemic amyloidosis in England: an epidemiological study. Br J Haematol. 2013;161:525–532. doi: 10.1111/bjh.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnellan E., Wazni O.M., Hanna M. Atrial fibrillation in transthyretin cardiac amyloidosis. J Am Coll Cardiol EP. 2020;6:1118–1127. doi: 10.1016/j.jacep.2020.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Feng D.L., Edwards W.D., Oh J.K. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation. 2007;116:2420–2426. doi: 10.1161/CIRCULATIONAHA.107.697763. [DOI] [PubMed] [Google Scholar]
- 21.Feng D.L., Syed I.S., Martinez M. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation. 2009;119:2490–2497. doi: 10.1161/CIRCULATIONAHA.108.785014. [DOI] [PubMed] [Google Scholar]
- 22.Donnellan E., Elshazly M.B., Vakamudi S. No association between CHADS-VASc score and left atrial appendage thrombus in patients with transthyretin amyloidosis. J Am Coll Cardiol EP. 2019;5:1473–1474. doi: 10.1016/j.jacep.2019.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Isath A., Correa A., Siroky G.P. Trends, burden, and impact of arrhythmia on cardiac amyloid patients: a 16-year nationwide study from 1999 to 2014. J Arrhythmia. 2020;36:727–734. doi: 10.1002/joa3.12376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollak A., Falk R.H. Left ventricular systolic dysfunction precipitated by verapamil in cardiac amyloidosis. Chest. 1993;104:618–620. doi: 10.1378/chest.104.2.618. [DOI] [PubMed] [Google Scholar]
- 25.Griffiths B.E., Hughes P., Dowdle R. Cardiac amyloidosis with asymmetrical septal hypertrophy and deterioration after nifedipine. Thorax. 1982;37:711–712. doi: 10.1136/thx.37.9.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubinow A., Skinner M., Cohen A.S. Digoxin sensitivity in amyloid cardiomyopathy. Circulation. 1981;63:1285–1288. doi: 10.1161/01.cir.63.6.1285. [DOI] [PubMed] [Google Scholar]
- 27.Mints Y.Y., Doros G., Berk J.L. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail. 2018;5:772–779. doi: 10.1002/ehf2.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Echt D.S., Liebson P.R., Mitchell L.B. Mortality and morbidity in patients receiving encainide, flecainide, or placebo: the cardiac arrhythmia suppression trial. N Engl J Med. 1991;324:781–788. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 29.Dan G.A., Martinez-Rubio A., Agewall S. Antiarrhythmic drugs-clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP) Europace. 2018;20:731–732. doi: 10.1093/europace/eux373. [DOI] [PubMed] [Google Scholar]
- 30.El-Am E.A., Dispenzieri A., Melduni R.M. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol. 2019;73:589–597. doi: 10.1016/j.jacc.2018.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loungani R.S., Rehorn M.R., Geurink K.R. Outcomes following cardioversion for patients with cardiac amyloidosis and atrial fibrillation or atrial flutter. Am Heart Jl. 2020;222:26–29. doi: 10.1016/j.ahj.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Mitrani L.R., De Los Santos J., Driggin E. Anticoagulation with warfarin compared to novel oral anticoagulants for atrial fibrillation in adults with transthyretin cardiac amyloidosis: comparison of thromboembolic events and major bleeding. Amyloid. 2020;28:30–34. doi: 10.1080/13506129.2020.1810010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reddy V.Y., Sievert H., Halperin J. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation a randomized clinical trial. J Am Med Assoc. 2014;312:1988–1998. doi: 10.1001/jama.2014.15192. [DOI] [PubMed] [Google Scholar]
- 34.Holmes D.R., Kar S., Price M.J. Prospective randomized evaluation of the watchman left atrial appendage closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 35.Barbhaiya C.R., Kumar S., Baldinger S.H. Electrophysiologic assessment of conduction abnormalities and atrial arrhythmias associated with amyloid cardiomyopathy. Heart Rhythm. 2016;13:383–390. doi: 10.1016/j.hrthm.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 36.Tan N.Y., Mohsin Y., Hodge D.O. Catheter ablation for atrial arrhythmias in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. 2016;27:1167–1173. doi: 10.1111/jce.13046. [DOI] [PubMed] [Google Scholar]
- 37.Black-Maier E., Rehorn M., Loungani R. Catheter ablation of atrial fibrillation in cardiac amyloidosis. Pacing Clin Electrophysiol. 2020;43:913–921. doi: 10.1111/pace.13992. [DOI] [PubMed] [Google Scholar]
- 38.Donnellan E., Wazni O., Kanj M., Elshazly M.B., Hussein A., Baranowski B. Atrial fibrillation ablation in patients with transthyretin cardiac amyloidosis. Europace. 2020;22(2):259–264. doi: 10.1093/europace/euz314. [DOI] [PubMed] [Google Scholar]
- 39.Donnellan E., Wazni O., Kanj M. Atrial fibrillation ablation in patients with transthyretin cardiac amyloidosis. Europace. 2020;22:259–264. doi: 10.1093/europace/euz314. [DOI] [PubMed] [Google Scholar]
- 40.Donnellan E., Wazni O.M., Saliba W.I. Prevalence, incidence, and impact on mortality of conduction system disease in transthyretin cardiac amyloidosis. Am J Cardiol. 2020;128:140–146. doi: 10.1016/j.amjcard.2020.05.021. [DOI] [PubMed] [Google Scholar]
- 41.Algalarrondo V., Dinanian S., Juin C. Prophylactic pacemaker implantation in familial amyloid polyneuropathy. Heart Rhythm. 2012;9:1069–1075. doi: 10.1016/j.hrthm.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 42.Rehorn M.R., Loungani R.S., Black-Maier E. Cardiac implantable electronic devices: a window into the evolution of conduction disease in cardiac amyloidosis. J Am Coll Cardiol EP. 2020;6:1144–1154. doi: 10.1016/j.jacep.2020.04.020. [DOI] [PubMed] [Google Scholar]
- 43.Donnellan E., Wazni O.M., Saliba W.I. Cardiac devices in patients with transthyretin amyloidosis: impact on functional class, left ventricular function, mitral regurgitation, and mortality. J Cardiovasc Electrophysiol. 2019;30:2427–2432. doi: 10.1111/jce.14180. [DOI] [PubMed] [Google Scholar]
- 44.Donnellan E., Wazni O.M., Hanna M. Primary prevention implantable cardioverter-defibrillators in transthyretin cardiac amyloidosis. Pacing Clin Electrophysiol. 2020;43:1401–1403. doi: 10.1111/pace.14023. [DOI] [PubMed] [Google Scholar]
- 45.Hamon D., Algalarrondo V., Gandjbakhch E. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol. 2016;222:562–568. doi: 10.1016/j.ijcard.2016.07.254. [DOI] [PubMed] [Google Scholar]
- 46.Hashimura H., Ishibashi-Ueda H., Yonemoto Y. Late gadolinium enhancement in cardiac amyloidosis: attributable both to interstitial amyloid deposition and subendocardial fibrosis caused by ischemia. Heart Vessels. 2016;31:990–995. doi: 10.1007/s00380-015-0658-0. [DOI] [PubMed] [Google Scholar]
- 47.Sayed R.H., Rogers D., Khan F. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J. 2015;36:1098–1105. doi: 10.1093/eurheartj/ehu506. [DOI] [PubMed] [Google Scholar]
- 48.Kristen A.V., Dengler T.J., Hegenbart U. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm. 2008;5:235–240. doi: 10.1016/j.hrthm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 49.Higgins A.Y., Annapureddy A.R., Wang Y. Survival following implantable cardioverter-defibrillator implantation in patients with amyloid cardiomyopathy. J Am Heart Assoc. 2020;73:694. doi: 10.1161/JAHA.120.016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim E.J., Holmes B.B., Huang S. Outcomes in patients with cardiac amyloidosis and implantable cardioverter-defibrillator. Europace. 2020;22:1216–1223. doi: 10.1093/europace/euaa094. [DOI] [PubMed] [Google Scholar]
- 51.Lin G., Dispenzieri A., Kyle R. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. 2013;24:793–798. doi: 10.1111/jce.12123. [DOI] [PubMed] [Google Scholar]
- 52.Varr B.C., Zarafshar S., Coakley T. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm. 2014;11:158–162. doi: 10.1016/j.hrthm.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 53.Adams D., Gonzalez-Duarte A., O’Riordan W.D. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. [DOI] [PubMed] [Google Scholar]
- 54.Benson M.D., Waddington-Cruz M., Berk J.L. Inotersen treatment for patients with hereditary transthyretin amyloidosis. N Engl J Med. 2018;379:22–31. doi: 10.1056/NEJMoa1716793. [DOI] [PubMed] [Google Scholar]
- 55.Benson M.D., Dasgupta N.R., Rissing S.M. Safety and efficacy of a TTR specific antisense oligonucleotide in patients with transthyretin amyloid cardiomyopathy. Amyloid. 2017;24:217–223. doi: 10.1080/13506129.2017.1374946. [DOI] [PubMed] [Google Scholar]
- 56.Solomon S.D., Adams D., Kristen A. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin-mediated amyloidosis: analysis of the APOLLO Study. Circulation. 2019;139:431–443. doi: 10.1161/CIRCULATIONAHA.118.035831. [DOI] [PubMed] [Google Scholar]
- 57.Waxman A.J., Clasen S., Hwang W.T. Carfilzomib-associated cardiovascular adverse events a systematic review and meta-analysis. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2017.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]