FIGURE 1.
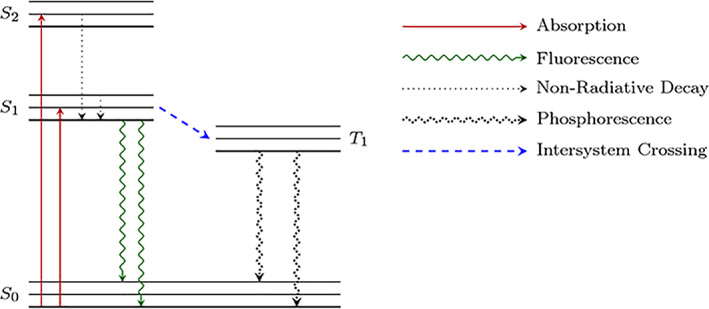
S0, S1 and S2 represent the ground state, first excited singlet state and second excited singlet state respectively and T1 represents the lowest excited triplet state. For fluorescence emission, electrons are excited from S0 to either S1 or S2. The electrons decay, non‐radiatively, to the ground level of S1 before decaying, radiatively to S0. For phosphorescence emission, which includes bioluminescence, electrons excited via chemical reaction transfer energy to T1 through intersystem crossing. The electrons then decay, radiatively, from the ground level of T1 to S0
