Abstract
Objective
At the time of multiple sclerosis (MS) diagnosis, identifying those at risk for poorer health-related quality of life and emotional well-being can be a critical consideration for treatment planning. This study aimed to test whether adverse childhood experiences predict MS patients’ health-related quality of life and emotional functioning at time of diagnosis and initial course of disease.
Methods
We recruited patients at the time of new MS diagnosis to complete self-report surveys at baseline and a one-year follow-up. Questionnaires included the Adverse Childhood Experiences (ACEs), as well as the MS Knowledge Questionnaire (MSKQ), the 36-Item Short Form Health Survey (SF-36), and Self-Management Screening (SeMaS).
Results
A total of n = 31 participants recently diagnosed with relapsing remitting MS (median EDSS = 1.0, age M = 33.84 ± 8.4 years) completed the study measures. The ACEs significantly predicted health-related quality of life (SF-36) at baseline (Adjusted R 2 = 0.18, p = 0.011) and follow-up (Adjusted R 2 = 0.12, p = 0.03), baseline scores on the SeMaS Depression scale (Adjusted R 2 = 0.19, p = 0.008), as well as follow-up scores on the SeMaS Anxiety (Adjusted R 2 = 0.19, p = 0.014) and SeMaS Depression (Adjusted R 2 = 0.14, p = 0.036) scales. Importantly, increased ACEs scores were predictive of increased anxiety at the one-year follow-up assessment, compared to baseline.
Conclusions
Childhood adversity predicts health-related quality of life and emotional well-being at time of MS diagnosis and over the initial course of the disease. Measured using a brief screening inventory (ACEs), routine administration may be useful for identifying patients in need of increased supportive services.
Keywords: chronic illness, coping, diagnosis, depression, anxiety, QoL, risk, trauma, well-being
Introduction
Multiple sclerosis (MS) is a chronic and progressive autoimmune disease of the central nervous system (CNS), causing neuroinflammation and neurodegeneration (Filippi et al., 2019; Lucchinetti et al., 2011). MS is typically diagnosed at a relatively young age, frequently in young to mid adulthood (Alroughani and Boyko, 2018; Tardieu et al., 2016; Tur and Thompson, 2015), and is up to 3 times more prevalent in females, compared to males (Briggs and Hill, 2020; Wallin et al., 2019; Walton et al., 2020). Receiving the diagnosis of an unpredictable, chronic, and progressive disease without cure may cause significant emotional distress (Patten et al., 2017; Werfel and Trettin, 2020). Inherent and learned coping mechanisms during the time of MS diagnosis and thereafter can ameliorate emotional reaction to diagnosis and disease (Calandri et al., 2017; Grech et al., 2018; Holland et al., 2019), resulting in reduced stress and better medication adherence (Bruce et al., 2010; Tarrants et al., 2011), as well as overall better outcomes (Strober, 2017; Van der Werf et al., 2003). On the other hand, maladaptive coping styles (e.g., avoidance) have been linked to increased depression and anxiety, and lower quality of life in individuals with MS (Goretti et al., 2010; Rabinowitz and Arnett, 2009; Roubinov et al., 2015).
Many individuals diagnosed with MS adjust to their illness and generally report satisfaction with life, regardless of level of disability, disease type, or disease progression (Chalk, 2007; Holland et al., 2019; Van Damme et al., 2016; Wilski et al., 2019; Yamout et al., 2013). Others, however, face a prolonged period of emotional distress, which can be long lasting, interfere with treatment adherence, and may exacerbate their disease (Patten et al., 2017; Werfel and Trettin, 2020). Identifying those who are at increased risk for poor coping and adjustment as early as at the time of diagnosis and providing them with the appropriate mental health services and support may have critical implications for their prognosis, as well as overall quality of life.
Childhood trauma and adversity have been established as major risk factors for mental and physical health problems in adulthood (Bellis et al., 2014; Felitti et al., 1998; Hughes et al., 2017; Sachs-Ericsson et al., 2016). Reduced psychological coping mechanisms related to multiple psychological, cognitive and psychosocial pathways are potentially the fundamental link between chronic stress and traumatic experiences in childhood and elevated emotional distress in adulthood (Nurius et al., 2015; Sachs-Ericsson et al., 2016). In the brain, exposure to stress was shown to lead to structural and functional alterations in the prefrontal cortex and limbic areas involved in stress-response (e.g., amygdala, hippocampus) by exerting its effects on neural plasticity mechanisms (McEwen and Gianaros, 2011; McEwen, 2012, 2016). At the endocrine level, chronic psychological stress has been linked to hyperactivation of the hypothalamic pituitary adrenal (HPA) axis (Miller et al., 2007; Heim et al., 2008) through elevated secretion of corticotropin-releasing factor (CRF), which can lead to dysregulation of the anti-inflammatory pathways (Bottasso, 2018; Chrousos, 1995). In addition, dysregulation of the HPA axis related to childhood stress and trauma has been linked to psychopathology in adulthood, including depression (Heim et al., 2008; Shea et al., 2005) and anxiety (Faravelli et al., 2012). The immune system is also sensitive to stress, and exposure to stress during childhood has been linked to increased inflammatory processes which can be long-lasting. For instance, it has been shown that children who experienced adverse events before the age of 8, had elevated inflammatory markers (interleukin-6; IL-6, C-reactive protein; CRP) in late childhood and early adolescence (ages 10–15) (Slopen et al., 2013). Elevated CRP, IL-6, and tumor necrosis factor-α (TNF-α) were also identified in adults who were exposed to adverse events in childhood, suggesting that these pro-inflammatory processes endure well into adulthood (Baumeister et al., 2016).
The Adverse Childhood Experiences (ACEs; Felitti et al., 1998) is a 10-item yes/no inventory developed to facilitate the study of these experiences on health outcomes. Resulting from a large epidemiologic study (n = 17,337) conducted by the U.S. health maintenance organization Kaiser Permanente and the Centers for Disease Control and Prevention, it includes the events that were associated with the greatest impact on health. The ACEs has since been well validated across a range of populations and shown to be highly predictive of behavioral and mental health status, including increased risk for developing depression and suicidality (Chapman et al., 2004; Felitti et al., 1998; Merrick et al., 2017; Schilling et al., 2007), as well as increased utilization of psychotropic medications (Anda et al., 2007). It has also been linked to increased risk of developing various medical conditions in adulthood, including obesity, heart disease, cancer, and lung disease (Dube et al., 2009; Felitti et al., 1998).
Adverse childhood experiences have also been linked to autoimmune disorders, with those experiencing two or more events in childhood at a higher risk for hospitalization with a diagnosis of an autoimmune disorder well into adulthood (Dube et al., 2009). ACEs scores were additionally associated with prevalence and severity of systemic lupus erythematosus (SLE) (DeQuattro et al., 2020) and rheumatic diseases (Luiz et al., 2018). However, the role of childhood trauma has not been well characterized in MS. Preliminary studies have found increased prevalence of self-reported childhood trauma in individuals with MS, compared to the general population, which was further associated with an increased relapse rate among those diagnosed with MS (Spitzer et al., 2012). Utilizing the ACEs, our group has previously reported increases linked to earlier age of onset and decreased estimated premorbid cognitive functioning (Shaw et al., 2017).
Further distinguishing the link between childhood adversity, health-related quality of life, and psychological functioning in MS may have critical implications for treatment planning and outcomes. Identifying those patients who are at an increased risk, as early as at the time of initial diagnosis, may allow the treatment team to promptly provide these individuals additional resources for emotional support, with the goal of improving coping and adjustment, ultimately leading to better prognosis and overall quality of life.
This is the first study to characterize the role of adverse childhood experiences in health-related quality of life and emotional well-being in individuals recently diagnosed with MS. Childhood trauma was measured through the ACEs, and its efficacy was examined in an effort to predict health-related quality of life and emotional functioning at the initial diagnosis, as well as at a one-year follow-up. In addition, previous studies suggested that increasing patients’ knowledge about their disease can mediate psychological reaction to MS diagnosis (Johnson, 2003) by empowering the patients and allowing them to assume a more active role in the medical decision making process (Colligan et al., 2017; Heesen et al., 2004, 2007). Thus, the predictive power of the ACEs was also compared to that of MS disease knowledge, as measured by the MS Knowledge Questionnaire (MSKQ). We hypothesized that the ACEs will significantly predict health-related quality of life and emotional functioning both at the time of diagnosis, as well as following one year, in individuals with MS. We further hypothesized that the ACEs will be a stronger predictor of these factors, relative to the MSKQ.
Methods
Participants
A total of 54 participants were recruited for this study, with n = 31 (ages 20 to 53, mean age = 33.84) completing all study measures. Demographic and clinical characteristics of the sample are summarized in Table 1. Participants were recruited and enrolled by the NYU Langone MS Comprehensive Care Center in New York, New York. All participants had a confirmed diagnosis of relapsing-remitting MS according to established criteria (Thompson et al., 2018). Eligibility criteria included ages between 18 and 65, without other major medical or psychiatric conditions, including seizure disorders, mood disorders, and psychotic disorders, to enable a more direct assessment of emotional reaction to MS diagnosis in our participants. In addition, all participants were able to understand and complete questionnaires written in English.
Table 1.
Sample characteristics.
| Participants (n = 31) | |
|---|---|
| Mean age (range) | 33.84 (20–53) |
| Mean years education (range) | 16.1 (12–20.5) |
| Percent female | 81% |
| Race n (%) | |
| Caucasian | 24 (77.4%) |
| African-American | 7 (22.5%) |
| Diagnosis n (%) | |
| RRMS | 31 (100%) |
| SPMS | 0 (0%) |
| PPMS | 0 (0%) |
| Not reported | 0 (0%) |
| Median EDSS (range) | 1.0 (0–6.5) |
All participants provided written informed consent to study procedures approved by the Institutional Review Board and Committee on Research Involving Human Subjects at NYU Langone Health, New York, New York (IRB approval number i16-00203), and in compliance with the Helsinki Declaration.
Materials
Baseline questionnaires were administered to participants within one month of the visit confirming their MS diagnosis. The survey was completed electronically on the HIPAA compliant NYU REDCap database and included the ACEs, MS Knowledge Questionnaire (MSKQ), Medical Outcomes Study Short Form–36 Items (SF-36), and Self-Management Screening (SeMaS). At one year following the baseline survey, participants were contacted by email or phone to complete the follow-up survey. The follow-up survey included the MSKQ, SF-36, and SeMaS scales.
Study Measures. The Adverse Childhood Experiences (ACEs) (Felitti et al., 1998). A 10-item validated, reliable, and widely used questionnaire assessing childhood exposure to different adverse experience (e.g., physical, verbal, or sexual abuse, mental illness or substance abuse in the nuclear family). The ACEs has at least modest test-retest reliability in healthy populations and an established record of robust association with health outcomes at the population level and within many specific disease categories (Bernstein et al., 2003; Felitti et al., 1998; Zanotti et al., 2018). Childhood exposure to each of the items on the scale is scored as one point (with binary Yes/No response to each item), with a maximum total score of 10. The higher the score on the ACE scale, the greater is the exposure to stressful and traumatic events in childhood. An ACEs score ≥ 4 represents an exponentially increased risk for developing negative physical and emotional outcomes in adulthood (Felitti et al., 1998). Thus, in the current study we used both continuous scoring of ACEs (i.e., the number of ACEs on a 0–10 scale), as well as categorical scoring with 3 groups; No ACEs (ACEs = 0), Low ACEs (ACEs = 1–3), and High ACEs (ACEs ≥4).
The MS Knowledge Questionnaire (MSKQ) (Giordano et al., 2010). The MSKQ comprises of 25 items (multiple choice questions) designed to test the participants overall knowledge of MS. It was shown to have good acceptability, internal consistency (Kuder–Richardson-20 = 0.76), and content validity (Giordano et al., 2010). Each correct answer to questions on the MSKQ is counted as one point, with a maximum total score of 25. Higher scores on the MSKQ reflect a wider extent of knowledge on MS.
The Medical Outcomes Study Short Form–36 Items (SF-36) (Stewart et al., 1988). A validated and widely used health status questionnaire assessing the effects of illnesses on emotional and physical well-being and quality of life, consisting of eight scales (with response categories vary between dichotomous Yes/No response and 3–5 point Likert scales). The SF-36 was demonstrated to have good validity and reliability (McHorney et al., 1993, 1994). Raw scores were computed for each of the scales and converted to z-scores based on normative data collected for the US population. An aggregated score for both the Physical Component Scale (PCS) and the Mental Component Scale (MCS) were calculated using the Z-scores for each subscale, multiplied by their weight and summed scores, according to the MSQLI SF-36 guidelines. Higher scores on the SF-36 represent better outcomes.
The Self-Management Screening (SeMaS) scale (Eikelenboom et al., 2013). The SeMaS is comprised of 27 questions derived from other validated questionnaires, which are grouped into seven subscales, assessing different aspects of emotional well-being. It was demonstrated to have good psychometric properties (positive predictive value = 41.5–77.8%, negative predictive value = 53.3–99.4%, Cronbach’s alpha = 0.56–0.87, and convergent construct validity of r2 = 0.317) (Eikelenboom et al., 2015). For the purpose of the current study, we looked at the SeMaS Anxiety and Depression subscales. Each item is rated on a 0 to 4 scale according to its frequency (Never/Sometimes/Often/Frequently/Always, with 0 being “never” and 4 being “always”). Higher scores on these scales represent higher levels of emotional distress.
Data analysis
Descriptive statistics were explored for the ACEs and MSKQ to characterize the sample. The predictive power of ACEs and MSKQ was examined using linear regression models. Baseline, follow-up, and change (follow-up minus baseline) scores on the SF-36, SeMaS Anxiety, and SeMaS Depression scales were entered as dependent variables. We predicted that higher ACEe scores would predict lower scores (i.e., worse outcome) on the SF-36, and higher scores on both the SeMaS Depression and Anxiety scales (i.e., greater levels of depression and anxiety), at both baseline and follow-up. We further predicted that higher ACEs scores would predict negative change on the SF-36 (i.e., reduced health-related quality of life) and positive change on both the SeMaS Depression and Anxiety scales (i.e., increased depression and anxiety) from baseline to follow-up.
In addition, an exploratory analysis (due to the small sample size) was conducted, stratifying the sample by ACEs severity, as it has been previously shown that an ACEs score ≥ 4 represents an exponentially increased risk for developing negative physical and emotional outcomes in adulthood (Felitti et al., 1998). Differences between the groups on baseline and follow-up outcome measures were tested with One-Way Analyses of Variance (ANOVA), and the Fisher’s Least Significant Difference (LSD) post hoc test was used for significant F-values. We predicted worse outcomes in participants with ACEs score of 4 or greater, compared to those with ACEs < 4.
Statistical analyses were performed using SPSS statistical package version 25.0 (IBM Corp, 2017).
Results
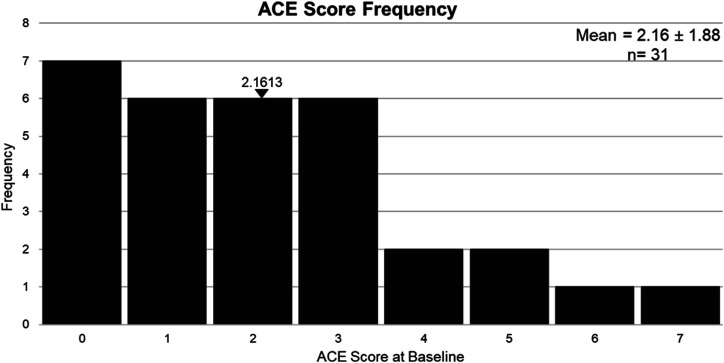
ACEs. Twenty-four out of the 31 (77%) participants in our sample reported at least one ACE, and 6 participants (19%) reported at least 4 ACEs, with mean score of 2.16 ± 1.88, ranging between 0 and 7 (Figure 1).
Figure 1.
Frequency distribution of ACE scores in our sample (n = 31).
MSKQ. In terms of knowledge of MS, mean score on the MSKQ at baseline was 8.81 ± 1.22, ranging between 6 and 11. At follow-up, MSKQ was similar (mean = 8.88 ± 0.95, range 7–10), with no significant differences between baseline and follow-up measures (as was indicated by a paired t-test; t = 0.15, p > 0.05), suggesting that in general, participants in our sample did not accumulate additional knowledge of the disease in the year between assessments.
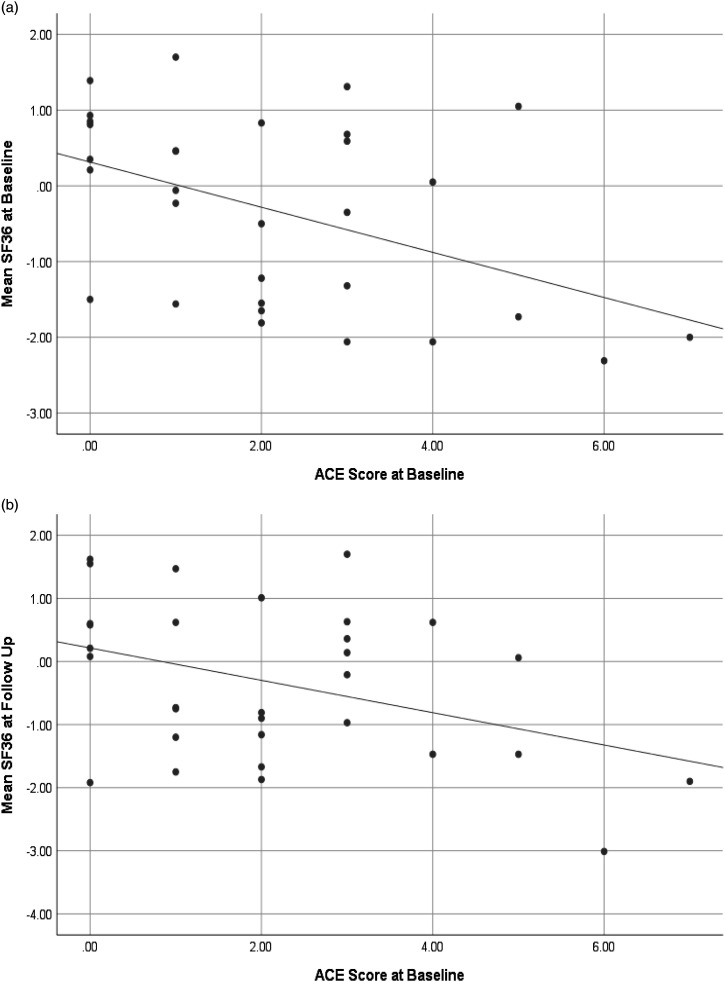
ACEs and MSKQ predictions of health-related quality of life and emotional distress at baseline. The predictive power of ACEs and MSKQ was examined using a linear regression model. Baseline SF-36 score, SeMaS Anxiety score, and SeMaS Depression score were entered as dependent variables. Assumptions of normality, linearity, homoscedasticity, and absence of multicollinearity were all met. Results indicated that scores on the ACEs significantly predicted SF-36 (Adjusted R 2 = 0.18, F = 7.45, p = 0.011; Figure 2) and SeMaS Depression (Adjusted R 2 = 0.19, F = 8.03, p = 0.008) scores. ACE did not significantly predict scores on the SeMaS Anxiety scale at baseline (Adjusted R 2 = 0.06, F = 3.02, p = 0.09). The MSKQ did not significantly predict any of these outcomes at baseline (SF-36, Adjusted R 2 = −0.01, F = 0.56, p = 0.46; SeMaS Depression, Adjusted R 2 = −0.01, F = 0.52, p = 0.47; SeMaS Anxiety, Adjusted R 2 = −0.02, F = 0.07, p = 0.80) (Table 2).
Figure 2.
ACE scores at baseline predict health-related quality of life (SF-36) at (a) baseline and (b) at a one-year follow-up.
Table 2.
Summary of results of regression analyses.
| Predictor | Outcome | Time/Measure | Adjusted R 2 | F | P |
|---|---|---|---|---|---|
| ACEs | SF-36 | Baseline | 0.18 | 7.45 | 0.01 a |
| SeMaS Depression | Baseline | 0.19 | 8.03 | 0.01 a | |
| SeMaS Anxiety | Baseline | 0.06 | 3.02 | 0.09 | |
| SF-36 | Follow-up | 0.12 | 5.19 | 0.03 a | |
| SeMaS Depression | Follow-up | 0.14 | 4.95 | 0.04 a | |
| SeMaS Anxiety | Follow-up | 0.19 | 7.0 | 0.01 a | |
| SF-36 | Change | −0.02 | 0.38 | 0.54 | |
| SeMaS Depression | Change | −0.01 | 0.79 | 0.38 | |
| SeMaS Anxiety | Change | 0.12 | 4.29 | 0.049 a | |
| MSKQ | SF-36 | Baseline | −0.01 | 0.56 | 0.46 |
| SeMaS Depression | Baseline | −0.01 | 0.52 | 0.47 | |
| SeMaS Anxiety | Baseline | −0.02 | 0.07 | 0.80 | |
| SF-36 | Follow-up | −0.01 | 0.70 | 0.41 | |
| SeMaS Depression | Follow-up | −0.04 | 0.03 | 0.87 | |
| SeMaS Anxiety | Follow-up | 0.03 | 0.09 | 0.77 | |
| SF-36 | Change | −0.03 | 0.00 | 0.96 | |
| SeMaS Depression | Change | −0.02 | 0.38 | 0.54 | |
| SeMaS Anxiety | Change | −0.01 | 0.85 | 0.36 |
aDenotes statistically significant values.
ACEs and MSKQ predictions of health-related quality of life and emotional distress at a one-year follow-up. The ACEs and MSKQ were additionally examined in a linear regression model using scores on the outcome measures at the follow-up visit as dependent variables. Assumptions of normality, linearity, homoscedasticity, and absence of multicollinearity were all met. Results indicated that scores on the ACEs significantly predicted SF-36 (Adjusted R 2 = 0.12, F = 5.19, p = 0.03; Figure 2), SeMaS Anxiety (Adjusted R 2 = 0.19, F = 7.0, p = 0.014) and SeMaS Depression (Adjusted R 2 = 0.14, F = 4.95, p = 0.036) scores at follow-up. The MSKQ did not significantly predict any of the outcome measures at follow-up (SF-36, Adjusted R 2 = −0.01, F = 0.70, p = 0.41; SeMaS Anxiety, Adjusted R 2 = −0.03, F = 0.09, p = 0.77; SeMaS Depression, Adjusted R 2 = −0.04, F = 0.03, p = 0.87) (Table 2).
ACEs and MSKQ predictions of change in health-related quality of life and emotional distress between baseline and one-year follow-up. The predictive power of ACEs and MSKQ was examined using a linear regression model. Change scores between baseline and follow-up assessments on the SF-36, SeMaS Anxiety, and SeMaS Depression were entered as dependent variables. Assumptions of normality, linearity, homoscedasticity, and absence of multicollinearity were all met. Results indicated that scores on the ACE significantly predicted change scores on the SeMaS Anxiety scale (Adjusted R 2 = 0.12, F = 4.29, p = 0.049), such that higher ACEs scores were associated with increased anxiety scores at follow-up compared to baseline (r = 0.39, p = 0.025). ACEs did not significantly predict change scores on the SF-36 scale (Adjusted R 2 = −0.02, F = 0.38, p = 0.54) or the SeMaS Depression scale (Adjusted R 2 = −0.01, F = 0.79, p = 0.38). The MSKQ did not significantly predict change scores on any of these outcomes (SF-36, Adjusted R 2 = −0.03, F = 0.00, p = 0.96; SeMaS Anxiety, Adjusted R 2 = −0.02, F = 0.38, p = 0.54; SeMaS Depression, Adjusted R 2 = −0.01, F = 0.85, p = 0.36) (Table 2).
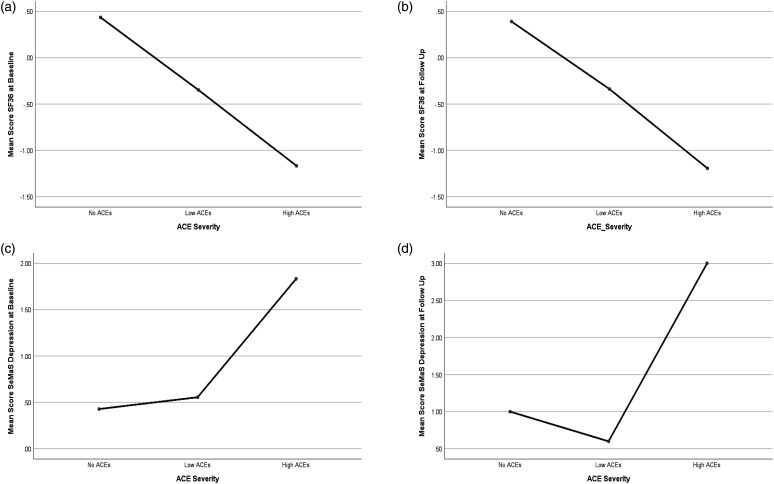
Levels of health-related quality of life and emotional state by ACEs severity. In an exploratory analysis, the sample was stratified by ACEs severity into three groups; no ACEs (ACEs = 0; n = 7), Low ACEs (ACEs = 1–3; n = 18) and High ACEs (ACEs ≥4; n = 6). Differences between the groups on baseline and follow-up outcome measures were tested with One-Way Analyses of Variance (ANOVA), and the Fisher’s Least Significant Difference (LSD) post hoc test was used for significant F-values. Results indicated significant effects of ACEs severity on baseline (F (2, 28) = 3.81, p = 0.03) and follow-up [F (2, 28) = 5.05, p = 0.02] SeMaS depression scores. A trend was identified in ACEs severity effects on SF-36 score at baseline [F (2, 28) = 3.07, p = 0.06] and at follow-up [F (2, 28) = 3.0, p = 0.066], but results did not reach statistical significance. Post hoc LSD tests revealed that for baseline SaMaS depression, High ACEs significantly differed from No ACEs (p = 0.02) and Low ACEs (p = 0.02), with greater depressive symptoms in the High ACEs (M = 1.83, SD = 1.47) relative to No ACEs (M = 0.43, SD = 1.13) and Low ACEs (M = 0.56, SD = 0.86); Low ACEs and No ACEs did not significantly differ (p = 0.79). Similarly, for follow-up SeMaS depression, High ACEs significantly differed from No ACEs (p = 0.03) and Low ACEs (p = 0.004), with greater depressive symptoms in the High ACEs (M = 3.0, SD = 2.35) relative to No ACEs (M = 1.0, SD = 2.0) and Low ACEs (M = 0.60, SD = 0.74); Low ACEs and No ACEs did not significantly differ (p = 0.58) (Figure 3). Finally, an independent sample t-test was utilized to examine differences in SF-36 between the No ACEs and High ACEs groups. Results indicated significant differences between the groups in both baseline SF-36 score [t (11) = 2.48, p = 0.03] and follow-up SF-36 score [t (11) = 2.28, p = 0.04], with poorer outcomes in the High ACEs at both baseline (High ACEs M = −1.17, SD = 1.38; No ACEs M = 0.43, SD = 0.94) and follow-up (High ACEs M = −1.19, SD = 1.33; No ACEs M = 0.39, SD = 1.18).
Figure 3.
One-way ANOVA with three levels of ACEs severity. ACEs effects on (a) baseline SF-36, p = 0.06, (b) follow-up SF-36, p = 0.066, (c) baseline SaMaS Depression, p = 0.03, and (d) follow-up SeMas Depression, p = 0.02.
Discussion
As hypothesized, our preliminary findings indicated that childhood trauma, as measured by the ACEs, significantly predicted poorer health-related quality of life and increased emotional distress in individuals with MS, both at the time of diagnosis, as well as at a one-year follow-up. These findings are consistent with those from previous studies in the general population demonstrating a strong link between ACEs and a range of detrimental medical, behavioral, and psychological outcomes in adulthood (Chapman et al., 2004; Dube et al., 2009; Felitti et al., 1998; Merrick et al., 2017, 2019), including a variety of autoimmune disorders (DeQuattro et al., 2020; Dube et al., 2009; Luiz et al., 2018). Our findings also expand on the initial research in individuals with MS, linking childhood trauma to an increased relapse rate (Spitzer et al., 2012) and ACEs with age of onset and cognitive functioning (Shaw et al., 2017). The current findings further demonstrate that childhood adversity, as measured by the ACEs, is associated with health-related quality of life and emotional well-being in MS, particularly around the time of diagnosis and the first year of adjustment to the disease.
The ACEs additionally predicted worsened anxiety over the first year post diagnosis. This discovery is of specific clinical importance, as it suggests that individuals who experienced adversity in childhood may have reduced capacity and skills for coping with adverse events in adulthood (e.g., being diagnosed with MS), resulting in increased emotional distress. Reduced psychological coping mechanisms related to multiple psychological, cognitive and psychosocial pathways are potentially the fundamental link between chronic stress and traumatic experiences in childhood, and elevated emotional distress in adulthood (Nurius et al., 2015; Sachs-Ericsson et al., 2016). Prolonged alterations in nervous, endocrine, and immune regulation in response to stressful events (Danese and McEwen, 2012; McEwen, 2012, 2016; Miller et al., 2007; Sachs-Ericsson et al., 2016) may also increase risks of psychopathology (Faravelli et al., 2012; Heim et al., 2008; Shea et al., 2005). Prior studies on emotion regulation in MS have shown that inherent and learned coping mechanisms can ameliorate emotional responses to diagnosis and the disease itself (Calandri et al., 2017; Grech et al., 2018; Holland et al., 2019), resulting in reduced stress and better medication adherence (Bruce et al., 2010; Tarrants et al., 2011), as well as an overall enhanced quality of life (Strober, 2017; Van der Werf et al., 2003). Thus, referral to appropriate mental health services and support at the initial diagnosis is potentially crucial in the prognosis of those patients who experience significant emotional distress, and whom psychological coping skills may be compromised. Our study demonstrates that childhood adversity, easily measured with the ACEs, could be essential in identifying patients who are at increased risk for poor emotional coping and adjustment, which in turn, can inform treatment planning and provision of appropriate supportive services.
Consistent with the larger literature (Felitti et al., 1998; Hillis et al., 2001), the current findings indicated that those who reported four or more ACEs had the poorest health-related quality of life and highest depression at both baseline and follow-up. Interestingly, our findings indicated that those who faced fewer than four adverse experiences in childhood were similar to participants who did not experience any childhood adverse events in terms of depression; while health-related quality of life had a more linear relationship with ACEs severity, such that greater ACEs scores were associated with poorer outcomes. These results suggest that while a cutoff score of four on the ACEs is appropriate for some aspects of emotional well-being (i.e., depression) in MS, those with fewer adverse childhood experiences may still experience reduced quality of life.
Finally, results showed that MS knowledge, measured by the MSKQ, did not have a significant role in the patient’s health-related quality of life or emotional distress at the time diagnosis or follow-up. Furthermore, MSKQ scores did not significantly differ between baseline and follow-up assessments in our sample, suggesting that participants did not actively seek additional MS-related knowledge in the first year following diagnosis. While it has been hypothesized that increasing patients’ knowledge about their disease may mediate psychological reaction to MS diagnosis (Johnson, 2003) by empowering the patients and allowing them to assume a more active role in the medical decision making process (Colligan et al., 2017; Heesen et al., 2004, 2007), it may also be associated with increased anxiety (Selinger et al., 2013). Thus, the role of MS knowledge in emotional coping and adjustment is not yet clear. In our study, participants did not actively seek to increase their knowledge between assessments and therefore, the role of MS knowledge in health-related quality of life and emotional functioning cannot be determined. Future studies, where participants actively increase their knowledge regarding their disease would be important in order to examine its effects on coping, adjustment, and health-related quality of life.
Study limitations include the relatively small size of our sample, which may limit statistical power and generalizability of the findings. In addition, while significant, our regression-model findings have small Adjusted R-square values and are not corrected for error rate, warranting caution in interpretation. While the current findings should be considered preliminary for these reasons, they clearly indicate the importance of screening for childhood trauma and adversity as early as at the time of diagnosis, and support the use of the ACEs as a valid measure for this purpose. Replication of these results with a larger sample would be important to further support the link between childhood adversity and psychological and health-related outcomes related to MS diagnosis and initial course of disease.
In addition, our sample is predominantly female and White. While this is broadly consistent with the general MS demographics in the United State (Amezcua and McCauley, 2020; Briggs and Hill, 2020; Wallin et al., 2019; Walton et al., 2020), there is data to suggest that rates of increase in incidents in MS are higher in Blacks, with greater burden of disease and functional limitations (Amezcua and McCauley, 2020). Our study also required participants to complete questionnaires in English, potentially further limiting the racial diversity of the sample. Thus, future studies should examine the effects of demographic factors, such as gender and race, on the experience of MS diagnosis in larger and more diverse samples.
Finally, as no measures of emotional functioning prior to MS diagnosis were available for this study, it is difficult to determine whether increased depressive symptoms and decreased health-related quality of life associated with ACEs at baseline are in fact related to MS diagnosis, or premorbid. However, increase in anxiety between baseline and follow-up measures associated with ACEs suggest increased emotional distress that is likely related, at least in part, to poorer emotional coping with the initial course of the disease. Moreover, this study utilized brief self-reported scales of depression and anxiety, that may not capture the full psychological extent of emotional adjustment to MS. Future investigation of the link between childhood adversity and emotional adjustment to MS utilizing a more comprehensive assessment of emotional reactivity and well-being would be important to fully characterize this important relationship.
Conclusion
Childhood adversity, simply measured with the ACEs, could be vital to characterize in MS, and may aid in identifying those patients who are at long-term risk for poorer health-related quality of life and increased emotional distress. Providing appropriate mental health resources and support to these individuals at the onset of the process may have critical implications for health and psychological outcomes by improving their treatment adherence, emotional well-being, and overall quality of life. Future work is needed to establish the utility of ACEs in our understanding of MS specifically and approached with more rigorous design and analyses. This continued line of study will allow for an ongoing evaluation of the ACEs as a predictor of mental and physical health outcomes for this disease group.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by supported by the Lourie Foundation, Inc.
ORCID iD
Leigh Charvet https://orcid.org/0000-0003-4429-9713
References
- Alroughani R, Boyko A. (2018) Pediatric multiple sclerosis: a review. BMC Neurology 18(1): 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amezcua L, McCauley JL. (2020) Race and ethnicity on MS presentation and disease course. Multiple Sclerosis Journal 26(5): 561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Brown DW, Felitti VJ, et al. (2007) Adverse childhood experiences and prescribed psychotropic medications in adults. American Journal of Preventive Medicine 32(5): 389–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister D, Akhtar R, Ciufolini S, et al. (2016) Childhood trauma and adulthood inflammation: a meta-analysis of peripheral C-reactive protein, interleukin-6 and tumour necrosis factor-α. Molecular Psychiatry 21(5): 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellis MA, Lowey H, Leckenby N, et al. (2014) Adverse childhood experiences: retrospective study to determine their impact on adult health behaviours and health outcomes in a UK population. Journal of Public Health 36(1): 81–91. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, et al. (2003) Development and validation of a brief screening version of the childhood trauma questionnaire. Child Abuse and Neglect 27(2): 169–190. [DOI] [PubMed] [Google Scholar]
- Bottasso O. (2018) Chronic Infections and the hypothalamus-pituitary-adrenal axis in the context of immune-mediated inflammation. Advances in Neuroimmune Biology 7(1): 79–89. [Google Scholar]
- Briggs FB, Hill E. (2020) Estimating the prevalence of multiple sclerosis using 56.6 million electronic health records from the United States. Multiple Sclerosis Journal 26(14): 1948–1952. [DOI] [PubMed] [Google Scholar]
- Bruce JM, Hancock LM, Arnett P, et al. (2010) Treatment adherence in multiple sclerosis: association with emotional status, personality, and cognition. Journal of Behavioral Medicine 33(3): 219–227. [DOI] [PubMed] [Google Scholar]
- Calandri E, Graziano F, Borghi M, et al. (2017) Coping strategies and adjustment to multiple sclerosis among recently diagnosed patients: the mediating role of sense of coherence. Clinical Rehabilitation 31(10): 1386–1395. [DOI] [PubMed] [Google Scholar]
- Chalk HM. (2007) Mind over matter: cognitive–behavioral determinants of emotional distress in multiple sclerosis patients. Psychology, Health & Medicine 12(5): 556–566. [DOI] [PubMed] [Google Scholar]
- Chapman DP, Whitfield CL, Felitti VJ, et al. (2004) Adverse childhood experiences and the risk of depressive disorders in adulthood. Journal of Affective Disorders 82(2): 217–225. [DOI] [PubMed] [Google Scholar]
- Chrousos GP. (1995) The hypothalamic–pituitary–adrenal axis and immune-mediated inflammation. The New England Journal of Medicine 332(20): 1351–1363. [DOI] [PubMed] [Google Scholar]
- Colligan E, Metzler A, Tiryaki E. (2017) Shared decision-making in multiple sclerosis. Multiple Sclerosis Journal 23(2): 185–190. [DOI] [PubMed] [Google Scholar]
- Danese A, McEwen BS. (2012) Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology and Behavior 106(1): 29–39. [DOI] [PubMed] [Google Scholar]
- DeQuattro K, Trupin L, Li J, et al. (2020) Relationships between adverse childhood experiences and health status in systemic lupus erythematosus. Arthritis Care and Research 72(4): 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Fairweather D, Pearson WS, et al. (2009) Cumulative childhood stress and autoimmune diseases in adults. Psychosomatic Medicine 71(2): 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom N, Smeele I, Faber M, et al. (2015) Validation of self-management screening (SeMaS), a tool to facilitate personalised counselling and support of patients with chronic diseases. BMC Family Practice 16(1): 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom N, van Lieshout J, Wensing M, et al. (2013) Implementation of personalized self-management support using the self-management screening questionnaire SeMaS; a study protocol for a cluster randomized trial. Trials 14(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faravelli C, Sauro CL, Godini L, et al. (2012) Childhood stressful events, HPA axis and anxiety disorders. World Journal of Psychiatry 2(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, et al. (1998) Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: The adverse childhood experiences (ACE) study. American Journal of Preventive Medicine 14(4): 245–258. [DOI] [PubMed] [Google Scholar]
- Filippi M, Brück W, Chard D, et al. (2019) Association between pathological and MRI findings in multiple sclerosis. Lancet Neurology 18(2): 198–210. [DOI] [PubMed] [Google Scholar]
- Giordano A, Uccelli MM, Pucci E, et al. (2010) The multiple sclerosis knowledge questionnaire: a self-administered instrument for recently diagnosed patients. Multiple Sclerosis Journal 16(1): 100–111. [DOI] [PubMed] [Google Scholar]
- Goretti B, Portaccio E, Zipoli V, et al. (2010) Coping strategies, cognitive impairment, psychological variables and their relationship with quality of life in multiple sclerosis. Neurological Sciences 31(2): 227–230. [DOI] [PubMed] [Google Scholar]
- Grech LB, Kiropoulos LA, Kirby KM, et al. (2018) Target coping strategies for interventions aimed at maximizing psychosocial adjustment in people with multiple sclerosis. International Journal of MS Care 20(3): 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesen C, Kasper J, Köpke S, et al. (2007) Informed shared decision making in multiple sclerosis—inevitable or impossible? Journal of the Neurological Sciences 259(1–2): 109–117. [DOI] [PubMed] [Google Scholar]
- Heesen C, Kasper J, Segal J, et al. (2004) Decisional role preferences, risk knowledge and information interests in patients with multiple sclerosis. Multiple Sclerosis Journal 10(6): 643–650. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Mletzko T, et al. (2008) The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology 33(6): 693–710. [DOI] [PubMed] [Google Scholar]
- Hillis SD, Anda RF, Felitti VJ, et al. (2001) Adverse childhood experiences and sexual risk behaviors in women: a retrospective cohort study. Family Planning Perspectives 33(5): 206–211. [PubMed] [Google Scholar]
- Holland D, Schlüter D, Young C, et al. (2019) Use of coping strategies in multiple sclerosis: Association with demographic and disease-related characteristics. Multiple sclerosis and related disorders 27: 214–222. [DOI] [PubMed] [Google Scholar]
- Hughes K, Bellis MA, Hardcastle KA, et al. (2017) The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health 2(8): e356–e366. [DOI] [PubMed] [Google Scholar]
- IBM Corp (2017) Released. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. [Google Scholar]
- Johnson J. (2003) On receiving the diagnosis of multiple sclerosis: managing the transition. . Multiple Sclerosis Journal 9(1): 82–88. [DOI] [PubMed] [Google Scholar]
- Lucchinetti CF, Popescu BF, Bunyan RF, et al. (2011) Inflammatory cortical demyelination in early multiple sclerosis. The New England Journal of Medicine 365(23): 2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiz APL, Antico HA, Skare TL, et al. (2018) Adverse childhood experience and rheumatic diseases. Clinical Rheumatology 37(10): 2863–2867. [DOI] [PubMed] [Google Scholar]
- McEwen BS. (2012) Brain on stress: how the social environment gets under the skin. Proceedings of the National Academy of Sciences 109(suppl 2): 17180–17185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. (2016) In pursuit of resilience: stress, epigenetics, and brain plasticity. Annals of the New York Academy of Sciences 1373(1): 56–64. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. (2011) Stress-and allostasis-induced brain plasticity. Annual Review of Medicine 62: 431–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr, Lu JR, et al. (1994) The MOS 36-item short-form health survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical Care 33(5): 40–66. [DOI] [PubMed] [Google Scholar]
- McHorney CA, Ware JE, Jr, Raczek AE. (1993) The MOS 36-item short-form health survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care 31(3): 247–263. [DOI] [PubMed] [Google Scholar]
- Merrick MT, Ford DC, Ports KA, et al. (2019) Vital signs: Estimated proportion of adult health problems attributable to adverse childhood experiences and implications for prevention—25 States, 2015–2017. MMWR. Morbidity and Mortality Weekly Report 68(44): 999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick MT, Ports KA, Ford DC, et al. (2017) Unpacking the impact of adverse childhood experiences on adult mental health. Child Abuse and Neglect 69: 10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. (2007) If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin 133(1): 25. [DOI] [PubMed] [Google Scholar]
- Nurius PS, Green S, Logan-Greene P, et al. (2015) Life course pathways of adverse childhood experiences toward adult psychological well-being: A stress process analysis. Child Abuse and Neglect 45: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten SB, Marrie RA, Carta MG. (2017) Depression in multiple sclerosis. International Review of Psychiatry 29(5): 463–472. [DOI] [PubMed] [Google Scholar]
- Rabinowitz AR, Arnett PA. (2009) A longitudinal analysis of cognitive dysfunction, coping, and depression in multiple sclerosis. Neuropsychology 23(5): 581. [DOI] [PubMed] [Google Scholar]
- Roubinov DS, Turner AP, Williams RM. (2015) Coping among individuals with multiple sclerosis: Evaluating a goodness-of-fit model. Rehabilitation Psychology 60(2): 162. [DOI] [PubMed] [Google Scholar]
- Sachs-Ericsson NJ, Rushing NC, Stanley IH, et al. (2016) In my end is my beginning: developmental trajectories of adverse childhood experiences to late-life suicide. Aging and Mental Health 20(2): 139–165. [DOI] [PubMed] [Google Scholar]
- Schilling EA, Aseltine RH, Gore S. (2007) Adverse childhood experiences and mental health in young adults: a longitudinal survey. BMC Public Health 7(1): 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinger CP, Lal S, Eaden J, et al. (2013) Better disease specific patient knowledge is associated with greater anxiety in inflammatory bowel disease. Journal of Crohn’s and Colitis 7(6): e214–e218. [DOI] [PubMed] [Google Scholar]
- Shaw MT, Pawlak NO, Frontario A, et al. (2017) Adverse childhood experiences are linked to age of onset and reading recognition in multiple sclerosis. Frontiers in Neurology 8: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea A, Walsh C, MacMillan H, et al. (2005) Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology 30(2): 162–178. [DOI] [PubMed] [Google Scholar]
- Slopen N, Kubzansky LD, McLaughlin KA, et al. (2013) Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology 38(2): 188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C, Bouchain M, Winkler LY, et al. (2012) Childhood trauma in multiple sclerosis: a case-control study. Psychosomatic Medicine 74(3): 312–318. [DOI] [PubMed] [Google Scholar]
- Stewart AL, Hays RD, Ware JE. (1988) The MOS short-form general health survey: reliability and validity in a patient population. Medical Care 26(7): 724–735. [DOI] [PubMed] [Google Scholar]
- Strober L. (2017) Personality in multiple sclerosis (MS): impact on health, psychological well-being, coping, and overall quality of life. Psychology, Health and Medicine 22(2): 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardieu M, Banwell B, Wolinsky JS, et al. (2016) Consensus definitions for pediatric MS and other demyelinating disorders in childhood. Neurology 87(suppl 2): S8–s11. [DOI] [PubMed] [Google Scholar]
- Tarrants M, Oleen-Burkey M, Castelli-Haley J, et al. (2011) The impact of comorbid depression on adherence to therapy for multiple sclerosis. Multiple Sclerosis International 2011: 271321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Banwell BL, Barkhof F, et al. (2018) Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurology 17(2): 162–173. [DOI] [PubMed] [Google Scholar]
- Tur C, Thompson AJ. (2015) Early accurate diagnosis crucial in multiple sclerosis. The Practitioner 259(1785): 21–27. [PubMed] [Google Scholar]
- Van Damme S, De Waegeneer A, Debruyne J. (2016) Do flexible goal adjustment and acceptance help preserve quality of life in patients with multiple sclerosis? International Journal of Behavioral Medicine 23(3): 333–339. [DOI] [PubMed] [Google Scholar]
- Van der Werf S, Evers A, Jongen PJ, et al. (2003) The role of helplessness as mediator between neurological disability, emotional instability, experienced fatigue and depression in patients with multiple sclerosis. Multiple Sclerosis Journal 9(1): 89–94. [DOI] [PubMed] [Google Scholar]
- Wallin MT, Culpepper WJ, Campbell JD, et al. (2019) The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology 92(10): e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton C, King R, Rechtman L, et al. (2020) Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS. Multiple Sclerosis Journal 26(14): 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werfel PB, Trettin L. (2020) Working with clients with multiple sclerosis. Journal of Health Service Psychology 46(1): 5–12. [Google Scholar]
- Wilski M, Gabryelski J, Brola W, et al. (2019) Health-related quality of life in multiple sclerosis: Links to acceptance, coping strategies and disease severity. Disability and Health Journal 12(4): 608–614. [DOI] [PubMed] [Google Scholar]
- Yamout B, Issa Z, Herlopian A, et al. (2013) Predictors of quality of life among multiple sclerosis patients: a comprehensive analysis. European Journal of Neurology 20(5): 756–764. [DOI] [PubMed] [Google Scholar]
- Zanotti DC, Kaier E, Vanasse R, et al. (2018) An examination of the test-retest reliability of the ACE-SQ in a sample of college athletes. Psychol Trauma 10(5): 559–562. [DOI] [PubMed] [Google Scholar]