Figure 6.
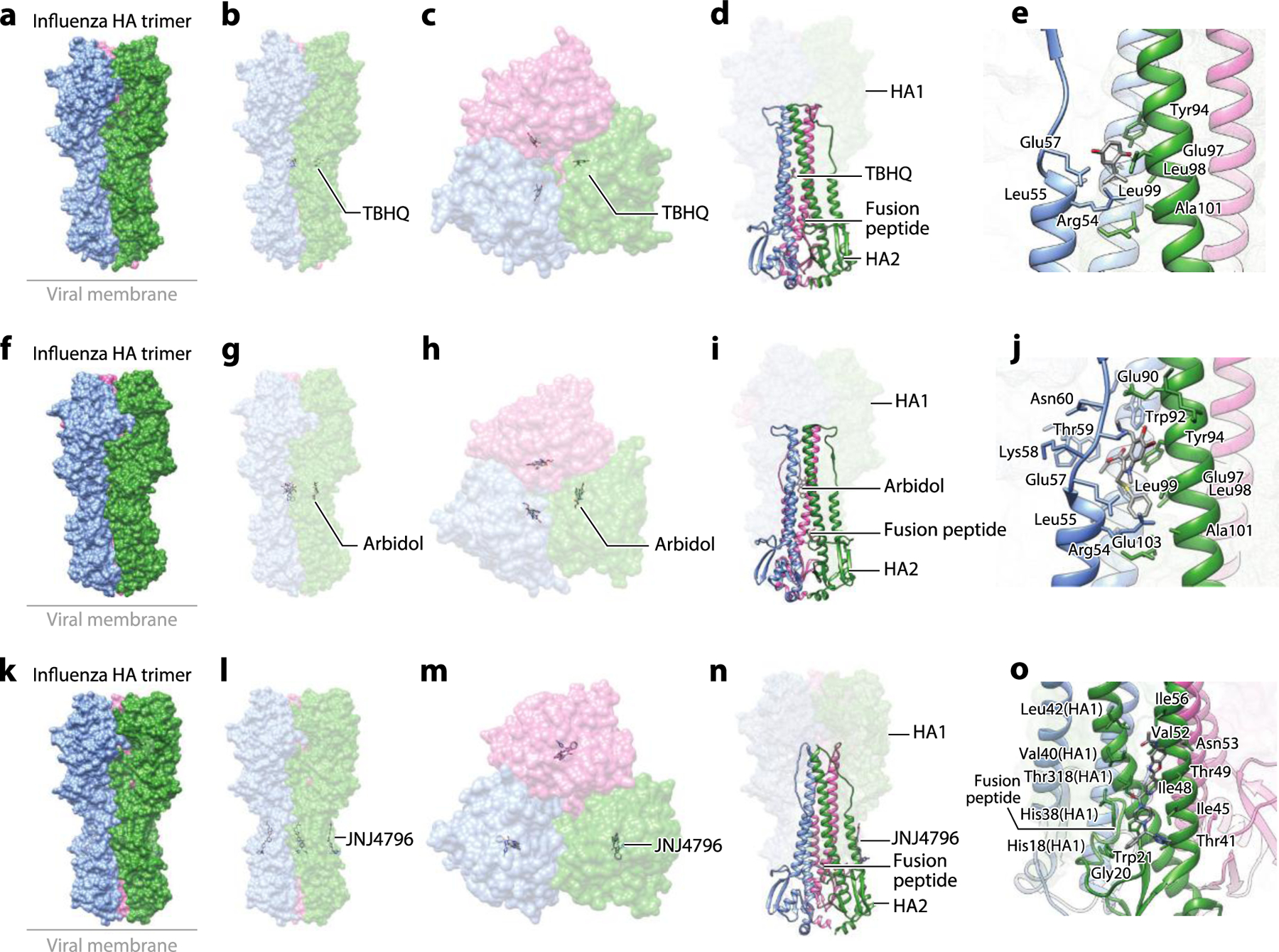
The structure of the influenza HA glycoprotein complexed with small-molecule inhibitors. (a) Structure of the influenza HA trimer complexed with TBHQ (PDB:3EYK). (b) The side view and (c) the top view of the complex structure showing binding of TBHQ in the inner hydrophobic cavity between protomers of the HA trimer. (d) Binding of TBHQ between the adjacent helixes is not proximal to the FP. (e) A zoomed-in view of binding of TBHQ to the hydrophobic cavity. (f) Structure of the influenza HA trimer complexed with arbidol (PDB:5T6S). (g) The side view and (h) the top view of the complex structure showing binding of arbidol in the inner hydrophobic cavity between protomers of the HA trimer. (i) Binding of arbidol between the adjacent helices is not proximal to the FP. (j) A zoomed-in view of arbidol bound in the hydrophobic cavity. (k) Structure of the influenza HA trimer complexed with JNJ4796 (PDB:6CF7). (l) The side view and (m) the top view of the complex structure showing binding of JNJ4796 to the hydrophobic groove at the interface of HA1 and HA2. (n) Binding of JNJ4796 is proximal to the FP. (o) A zoomed-in view of binding of JNJ4796 to the hydrophobic groove at the interface of HA1 and HA2. Abbreviations: FP, fusion peptide; HA, hemagglutinin; TBHQ, tertiary butylhydroquinone. Structural graphics were generated with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311 (166).
