Figure 7.
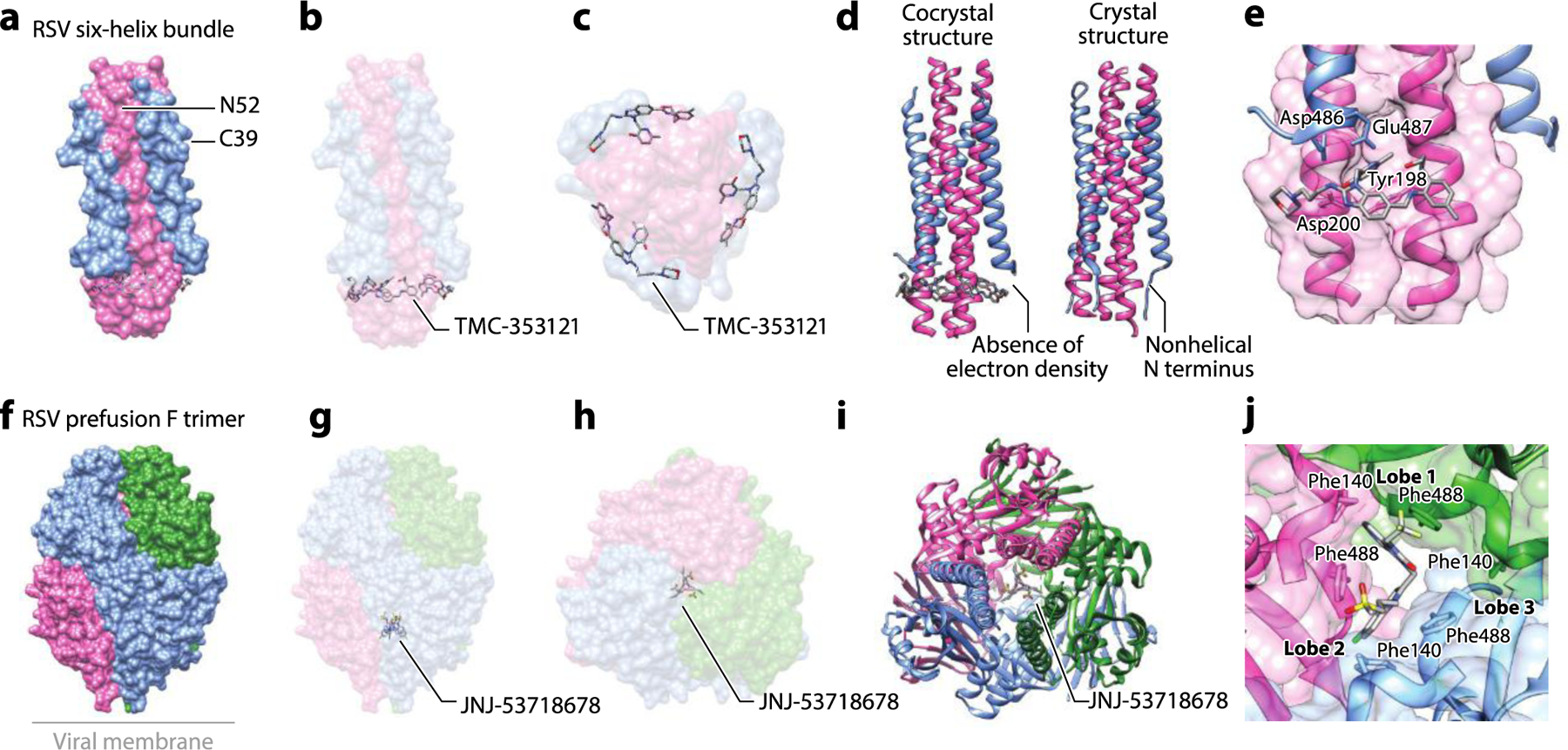
The structure of the RSV F glycoprotein complexed with small-molecule inhibitors. (a) Structure of the RSV six-helix bundle formed by N52 (HR1) and C39 (HR2) peptides with TMC-353121 (PDB:3KPE). (b) The side view and (c) the bottom view of the complex showing binding of TMC-353121 in the HR1 pocket. (d) Binding of TMC-353121 was proposed to distort the six-helix bundle due to absence of electron density at the N-terminal, nonhelical portion of the protein. The crystal structure of the native six-helix bundle (PDB:1G2C) is shown for comparison (19, 59). (e) A zoomed-in view of binding of TMC-353121 to the HR1 pocket. (f) The structure of DS-Cav1, a stabilized RSV prefusion F trimer, with JNJ-53718678 (PDB:5KWW). (g) The side view and (h) the top view of the complex shows JNJ-53718678 in the central cavity. (i) The binding of JNJ-53718678 in the central cavity was shown to stabilize the prefusion F. (j) A zoomed-in view of binding of JNJ-53718678 to two lobes at the interface of the F protein trimer in the central cavity and contacts made to Phe140 and Phe488. Abbreviations: HR, heptad repeat; RSV, respiratory syncytial virus. Structural graphics were generated with UCSF Chimera, developed by the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco, with support from NIH P41-GM103311 (166).
