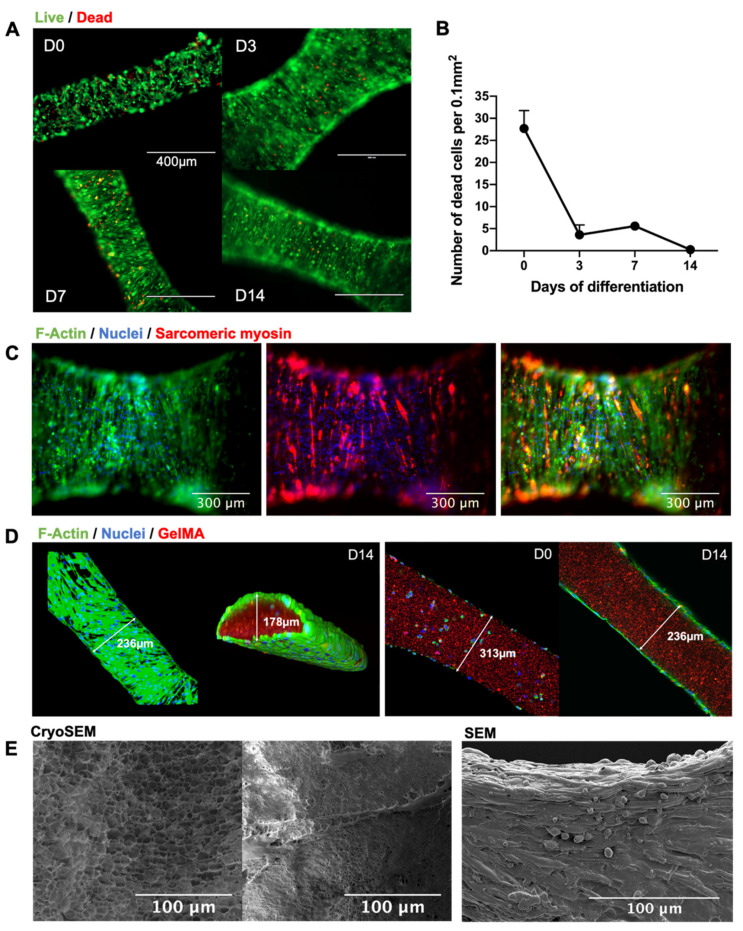
Figure 3.
Characterizing myoblast behavior in bioprinted fibers. (A) Live (green) and dead (red) cell stains were performed at days 0, 3, 7, and 14 of differentiation after bioprinting. Given the nature of myoblast fusion, live cells were not individually counted, although, qualitatively, the live stain revealed dense myofiber formation. (B) Cell death was reported as a cell count per 0.1 mm2 of printed fiber. There was a peak in cell death at day 0, as would be expected due to shear stress during extrusion, followed by a rapid recovery from day 3 to day 14. Error bars represent standard deviation. (C) Cells were stained for F-actin (green), sarcomeric myosin (red), and DNA (blue). The emergence of myosin-positive fibers is a marker of maturing contractile mechanisms within the myofiber. (D) Confocal imaging of cells demonstrated migration from within the GelMA to the boundary of the material, where they fused into multinucleated myotubes on the material surface. (E) CryoSEM of bioprinted GelMA showed the nature of myofiber adherence without surface microgrooving or patterning that might have influenced the direction of cell growth. SEM demonstrated a dense multilayered myotube culture on the printed GelMA fibers.