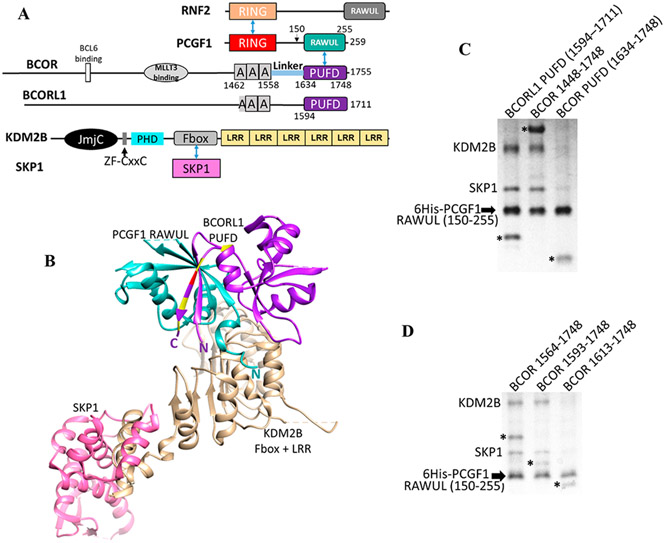
Figure 1.
PRC1.1, BCOR, and BCORL1. (A) Domain structure of PRC1.1 components. Blue arrows indicate direct protein–protein interactions between domains. The “A” within BCOR and BCORL1 is an ankyrin domain. (B) Interaction of the PCGF1 RAWUL/BCORL1 PUFD with KDM2B17 (PDB entry 5JH5) showing the importance of the N-terminus of PCGF1 RAWUL and both termini of the BCORL1 PUFD (termini labeled N and C). Residue positions where ITDs occur within BCOR are colored yellow and red at the equivalent positions on the BCORL1 structure. The red residue is the site of the ITD used in this study. (C and D). PCGF1 RAWUL [residues 150–255 with an N-terminal hexahistidine (6His) tag] was co-expressed in bacteria with KDM2B/SKP1 and different BCOR proteins. Components were assembled on the Ni2+ resin via the 6His tag on PCGF1 RAWUL. The KDM2B used in all experiments in this study contains the Fbox and the LRR domains (residues 1059–1336). Full-length SKP1 binds the Fbox domain of KDM2B and was required for KDM2B stabilization. BCORL1 and BCOR proteins in the gels are denoted with an asterisk. (C) Comparison between the PUFDs of BCORL1 (lane 1, positive control) and BCOR (lane 3). The presence of the BCOR band in lane 3 indicates the ability of the BCOR PUFD to dimerize with PCGF1. The absence of KDM2B/SKP indicates the inability to assemble the tetramer. Though the BCORL1 and BCOR PUFDs have similar numbers of residues, the difference in migration for the BCOR and BCORL1 PUFDs can be ascribed to the BCOR PUFD being slightly smaller in mass (13.6 kDa) than BCORL1 PUFD (14.0 kDa) and having a lower pI (4.59 for the BCOR PUFD vs 5.26 for the BCORL1 PUFD). (D) Residues N-terminal to the BCOR PUFD are required for assembly with KDM2B. When BCOR 1593–1748 is co-expressed (lane 2), a positive assembly signal is indicated by the presence of KDM2B/SKP1, which is absent when BCOR 1613–1748 (lane 3) or BCOR 1634–1748 (C, lane 3) is expressed. Thus, BCOR 1593–1748 is necessary and sufficient for KDM2B assembly. All expression constructs were identical except for BCOR. Equal amounts of the lysate of bacteria expressing all components were introduced onto Ni2+ resin. The resin was washed; equal amounts of the eluted contents were subjected to SDS–PAGE.