Figure 5.
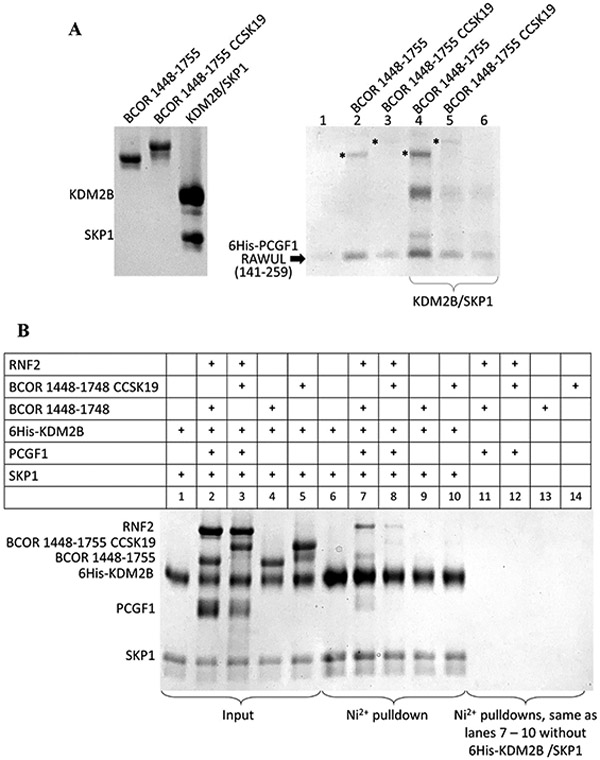
BCOR ITD disrupts PRC1.1 assembly. SDS–PAGE of Ni2+ affinity pull-down assays. (A) SDS–PAGE of the purified proteins (input) used for incubation with the 6His-PCGF1 RAWUL (140–255)-expressing bacterial lysate (left). SDS–PAGE of the Ni2+ affinity pull-down assay (right). Equal volumes of the bacterial lysate that expressed 6His-PCGF1 RAWUL were incubated with purified, non-6His-tagged proteins (labeled above the lanes and shown in the left gel) to a final concentration of 3 μM for 30 min prior to binding to the Ni2+-sepharose beads. Washing and elution were the same as described above. Because equal amounts of bacterial lysates were used for each of the lanes, any enrichment observed in the SDS–PAGE indicates a mutual stabilization of the entire assembly bound to the Ni2+-sepharose resin. For lanes 4–6, the purified KDM2B/SKP1 dimer (final concentration of 3 μM) was also added in addition to the proteins indicated above the gel. An asterisk indicates the BCOR proteins. (B) All proteins used were not from lysates but rather from purified protein solutions prepared at a final concentration of 5 μM in 120 μL of 50 mM Tris (pH 8.0), 200 mM NaCl, 50 mM imidazole (pH 7.5), and 10 mM βME and allowed to incubate at room temperature for 30 min. The protein solutions were then introduced onto 17 βL of Ni2+-sepharose beads, washed, and eluted as described above. The assembled proteins were immobilized on the Ni2+ column via the hexahistidine (6His)-tagged KDM2B/SKP1 dimer. Lanes 6–10 show elutions of the mixtures shown in lanes 1–5, respectively. The lower intensity of the BCOR band in lane 7 compared to the other pull-down figures presented in this study is likely the result of the alternative, non-optimal orientation of the Ni2+ immobilization utilized in this experiment where the hexahistine tag is at the N-terminus of KDM2B. The other pull-down experiments utilized the hexahistine tag on PCGF1 RAWUL.
