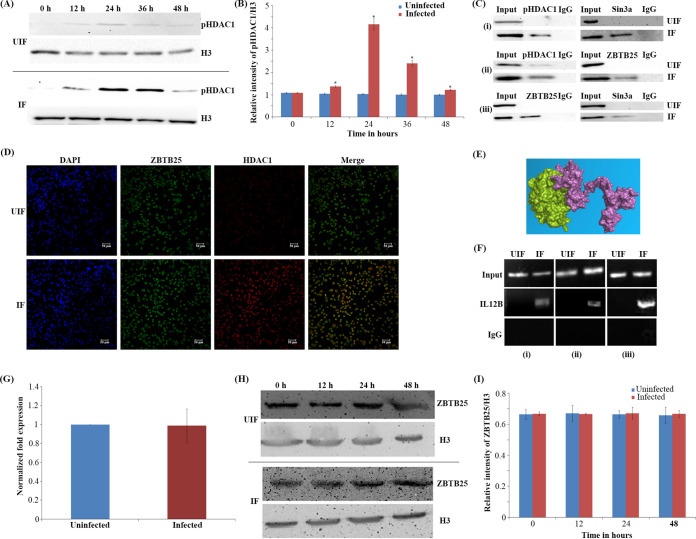
FIG 1.
Phosphorylated HDAC1 interacts with ZBTB25, and they are recruited to the IL-12B promoter. (A) Time course of levels of phosphorylated HDAC1 in M. tuberculosis-infected macrophages. pHDAC1 levels in uninfected (UIF) and infected (IF) samples. (B) Densitometric analysis of pHDAC1 bands normalized with that of histone H3. Each value represents mean ± SD from triplicate measurements. *, significantly different from UIF sample; P ≤ 0.05. (C) Coimmunoprecipitation analysis of the association between ZBTB25 and Sin3a-HDAC1 complex. Whole-cell lysates were immunoprecipitated with the antibodies against (i) ZBTB25, (ii) Sin3a, and (iii) HDAC1. IgG was used as the negative control. Immunocomplexes were then probed with antibodies as indicated. (D) Immuno-cytochemical imaging shows HDAC1 colocalizes with ZBTB25 inside the nucleus of macrophages infected with M. tuberculosis. The cells were visualized by confocal microscopy at 24 hpi. (E) Docking analysis shows that HDAC1 can interact with ZBTB25. (F) Status of ZBTB25, HDAC1, and Sin3a recruitment on the IL-12B promoter by ChIP. ChIP with (i) ZBTB25, (ii) HDAC1, and (iii) Sin3a antibodies. (G) Status of expression of ZBTB25 in macrophages upon M. tuberculosis infection by real-time PCR; (H) protein levels by Western blotting. (I) Densitometric analysis of ZBTB25 bands normalized with that of histone H3. Each value represents mean ± SD from triplicate measurements.