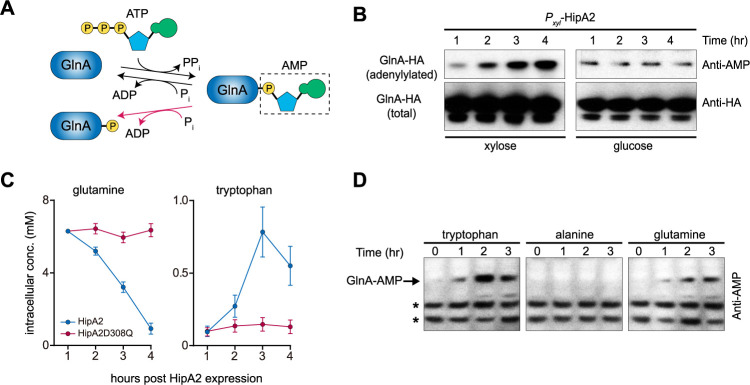
FIG 4.
HipA2 triggers an imbalance of intracellular free tryptophan and glutamine by stimulating GlnA adenylylation. (A) Schematic representation of GlnA adenylylation and deadenylylation. GlnA deadenylylation can be achieved by both hydrolysis (black arrow) and phosphorolysis (red arrow). (B) Stimulation of GlnA adenylylation by HipA2 analyzed by immunoprecipitation. Cells expressing HA-GlnA controlled by its native promoter and HipA2 controlled by the inducible xylose promoter were grown in PYE + glucose to exponential phase. Cells were collected, washed, and resuspended in PYE + xylose or PYE + glucose for induction or repression of HipA2 expression, respectively. Samples were collected at each indicated time point for immunoprecipitation using anti-HA magnetic beads and analyzed by SDS-PAGE and immunoprecipitation with either anti-AMP or anti-HA antibody. (C) Quantification of intracellular levels of free tryptophan and glutamine upon HipA2 activation. Strains harboring a chromosomal copy of Pxyl-hipA2 or Pxyl-hipA2D308Q (kinase-dead mutant) were grown in PYE + glucose to the exponential phase. Cells were collected, washed, and resuspended in PYE + xylose for HipA2 induction. Samples were collected at each indicated time point for free amino acid quantification, as described in Materials and Methods. (D) Comparison of GlnA adenylylation levels upon exposure to exogenous amino acids. Wild-type Caulobacter crescentus was grown in M2G medium to the exponential phase, and the culture was divided into three equal aliquots. The indicated amino acids were added to each aliquot, and cells were collected each hour for a period of 3 h. Lysates were prepared from each sample and analyzed by SDS-PAGE and immunoblotting with anti-AMP antibody. Due to the limited number of proteins that are adenylylated in Caulobacter (indicated by asterisks), we were able to compare levels of GlnA adenylylation in response to amino acid treatment.