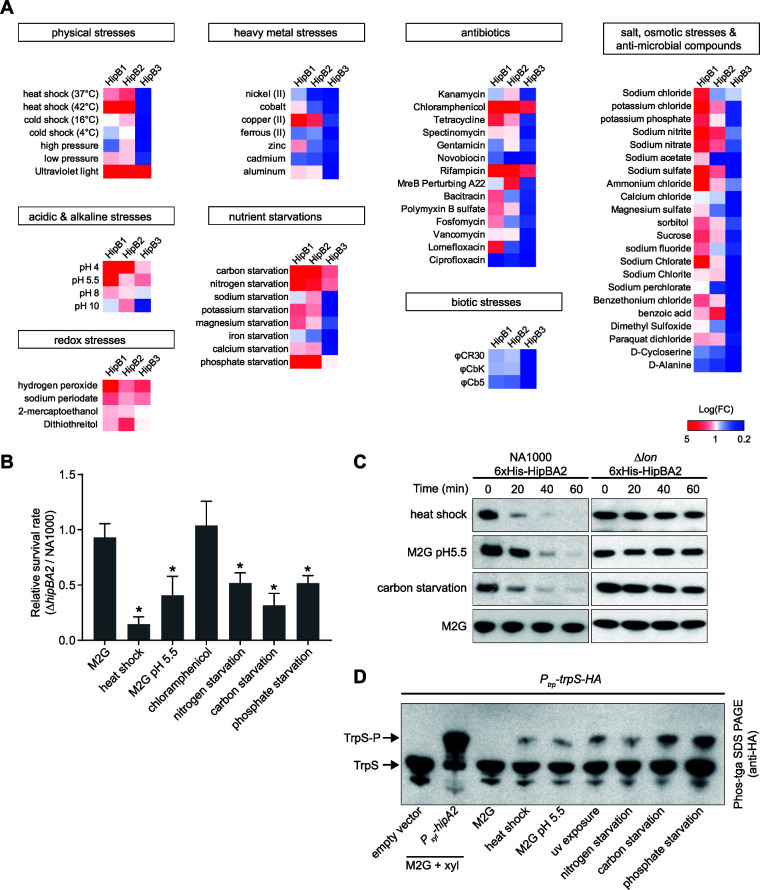
FIG 6.
Caulobacter utilizes the stringent response to cope with multiple stresses by activation of HipA2 toxin. (A) Screening of environmental cues required for toxin activation in wild-type Caulobacter crescentus. Cultures were exposed to 69 different stress conditions and tested for their ability to induce upregulated transcription of hipB genes by qRT-PCR. The log-fold change [Log(FC)] of hipB1, hipB2, and hipB3 transcription in strains challenged by those stresses was plotted in a heat map. The category of stress conditions used for this study is indicated, and specific conditions, agents, and antibiotics are listed in Table S2. (B) Survival rates of the ΔhipBA2 mutant relative to the wild-type strain. Cells were grown to the exponential phase from approximately the same starting density. Cultures were then challenged with indicated stresses for 30 min (see Table S2 for detailed information) followed by the addition of kanamycin (5 μg/ml) for another 4 h. Results are shown as percent survival rate by comparison to untreated cells prior to the addition of the antibiotic. Mean and SD from one batch performed with three biological replicates (n = 6) are plotted. An asterisk indicates a significant difference (P < 0.05) by two-tailed Student’s t test. (C) The HipB2 protein is degraded by the Lon protease under conditions of exposure to heat shock, low pH, and carbon starvation. Wild-type (WT) and Δlon strains harboring a hipBA2 operon (expressing 6×His-hipB and native hipA) on a plasmid were grown in M2G to exponential phase. Cells were then suspended in the medium conferring the indicated stresses with an additional supplement of chloramphenicol (200 μg/ml) to shut off protein synthesis. Samples were collected every 20 min, and protein levels were monitored by immunoblot assays using anti-His antibody. 6×His-hipB was stable in Δlon cells. (D) In vivo phosphorylation of TrpS under the challenge of various stresses. Cells expressing TrpS-HA were treated with the indicated stresses for 3 h. Whole-cell lysates were analyzed by Phos-tag SDS-PAGE and immunoblotting with anti-HA antibody. Samples containing an empty vector or expressing HipA2 served as negative and positive controls, respectively.