Thoracic aortic aneurysm and dissection (TAAD) is a highly lethal aortic disease. Administration of a lysyl oxidase inhibitor, β-aminopropionitrile (BAPN) fumarate, induces medial degeneration of aorta and results in TAAD formation in mice, characterizing by local dilation of the thoracic aortic wall with a maximum external diameter of >50% compared with the adjacent intact part of the aorta, a tear in the intimal layer of the aorta, extracellular matrix degradation, and inflammation. The pathogenesis of TAAD involves both genetic mutations such as FBN1, LOX, MYH11, and COL3A1 and environmental factors including aging, hypertension, and inflammation. Of note, 2 recent clinical studies indicate that the application of fluoroquinolone antibiotics increases the risk of TAAD.1,2 The cause-effect of fluoroquinolone antibiotics to TAAD is further confirmed by supplementation of ciprofloxacin to Ang II (angiotensin II)–infused mice.3 This alarming observation has raised concern in patients with risk for developing TAAD. Whether other clinical applicable drugs also increase the risk on TAAD progression or rupture?
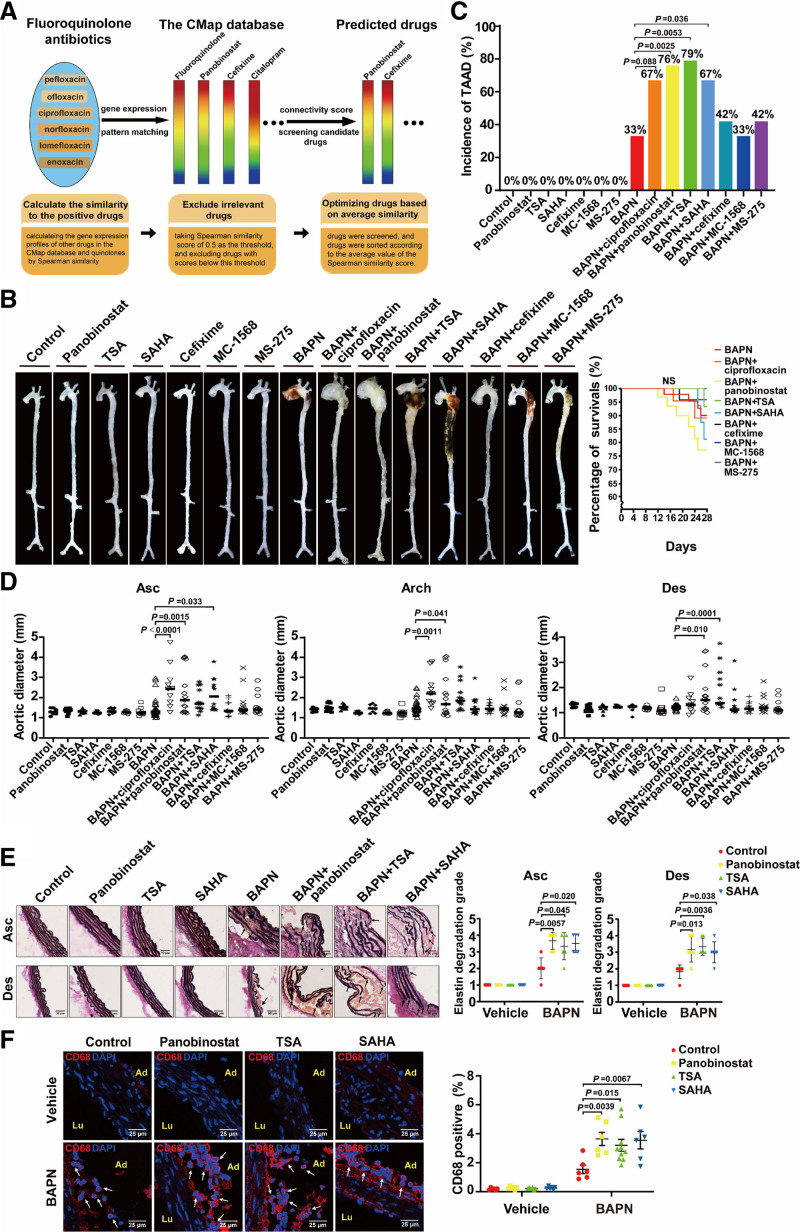
The Connectivity Map has provided a data-driven and systematic approach for discovering associations among chemicals, genes, and diseases based on genome-wide expression profiling.4 In principle, drugs with similar gene expression signatures may share similar mechanisms, chemical and physiological processes, and efficacies for similar diseases. By using 6 different fluoroquinolone antibiotics including pefloxacin, ofloxacin, ciprofloxacin, norfloxacin, lomefloxacin, and enoxacin as baits, we found that panobinostat and cefixime exhibited high degree of similarity with fluoroquinolones based on the Spearman correlation score by the Connectivity Map sorting algorithm (Figure [A]). Panobinostat is a pan-HDAC (histone deacetylase) inhibitor clinically applied for multiple myeloma therapy, and its Spearman similarity score was 0.577. Cefixime is a third-generation cephalosporin antibiotic, and its Spearman similarity score was 0.565. We examined whether these two drugs exhibited deleterious effects on TAAD in a BAPN-induced mouse model. C57BL/6J male mice aged 3 weeks were fed a normal laboratory diet and administered freshly prepared BAPN solution dissolved in the drinking water (0.5 g/kg per day) for consecutive 28 days. The mice were randomly divided into 7 groups: control, panobinostat, cefixime, BAPN, BAPN+ciprofloxacin, BAPN+panobinostat, and BAPN+cefixime. After 28 days, panobinostat and cefixime alone had no obvious changes compared with the control group. BAPN administration caused 33% (12/36) incidence of TAAD, including 11% (4/36) rupture and premature death in mice. Consistent with the previous report in which ciprofloxacin exacerbated TAAD in Ang II–infused mice,3 the application of ciprofloxacin in mice showed an increasing trend of the incidence of BAPN-induced TAAD (67% [8/12; P=0.088] incidence), including 17% (2/12; P=0.22) rupture and premature death in mice, and used it as a positive control. Intriguingly, the incidence of TAAD in mice of the BAPN+panobinostat group was increased to 76% (16/21; P=0.0025), including 29% (6/21; P=0.082) rupture and premature death, compared with the BAPN group. In contrast, cefixime (42% [5/12; P=0.73] incidence) did not aggravate BAPN-induced TAAD in mice and had no rupture and premature death (Figure [B and C]). Furthermore, the thoracic aortas from the mice of the BAPN+panobinostat group were more significantly expanded compared with the BAPN group (BAPN versus BAPN+panobinostat: ascending aortas, 1.47±0.48 versus 2.27±1.09 mm, P=0.0015; aortic arch, 1.54±0.34 versus 2.09±1.06 mm, P=0.041; descending aortas, 1.24±0.12 versus 1.75±0.80 mm, P=0.010; Figure [D]). Meanwhile, panobinostat aggravated elastin degradation and macrophage infiltration in aortic walls of TAAD induced by BAPN, as evidenced by elastic Van Gieson staining and immunofluorescent staining of CD68 (biomarker of macrophages), respectively (Figure [E and F]).
Figure.
pan-HDAC (histone deacetylase) inhibitors increase susceptibility of thoracic aortic aneurysm. A, The Connectivity Map (CMap) schematic diagram and the rational of drug screening from Connectivity Map database. The rainbow represents gene expression signature in response to the certain drug. Similar rainbow patterns represent the drugs have similar gene expression profiles. B–F, Three-week-old C57BL/6J male mice were administered vehicle (n=9), panobinostat (20 mg/kg 3 times per wk, n=12), TSA (trichostatin A; 0.6 mg/kg 3 times per wk, n=9), SAHA (vorinostat; 25 mg/kg daily for 14 d, n=9), cefixime (100 mg/kg per d, n=9); MC-1568 (50 mg/kg 3 times per wk, n=9), MS-275 (20 mg/kg 3 times per wk, n=9), BAPN (β-aminopropionitrile; 0.5 g/kg per d, n=36), BAPN+ciprofloxacin (100 mg/kg per d, n=12), BAPN+panobinostat (20 mg/kg 3 times per wk, n=21), BAPN+TSA (0.6 mg/kg 3 times per wk, n=14), BAPN+SAHA (25 mg/kg daily for 14 d, n=15), BAPN+cefixime (100 mg/kg per d, n=12), BAPN+MC-1568 (50 mg/kg 3 times per wk, n=15), or BAPN+MS-275 (20 mg/kg 3 times per wk, n=12) for 28 d. All mice were given both i.p. and i.g. administration simultaneously. For i.p. administration of panobinostat, TSA, SAHA, and MS-275, we also performed the vehicle i.g. administration. Conversely, the vehicle i.p. administration was performed in mice with i.g. administration of cefixime, ciprofloxacin, and MC-1568. The control- and BAPN-only groups were given both i.p. and i.g. administrations of vehicle. The body weight and the water intake of mice were measured every 2 d. The volume of water intake were ≈4.23 mL/d and 2.25 mL/d per mouse in control and BAPN group, respectively. The amount of water intake was comparable among all groups with BAPN administration, indicating BAPN was administered equally to mice. B, Representative ex vivo morphology of the aortas from the above 15 groups. C, Incidence of TAAD (thoracic aortic aneurysm and dissection; upper). Statistical analysis performed by Fisher exact test. Survival curve of mice with the indicated administration (lower). Statistical analysis performed by Log-rank test for Kaplan-Meier curve. D, Quantification of maximal aortic diameters measured ex vivo. Statistical analysis performed by 1-way ANOVA. E, Representative images of elastin Van Gieson staining of aortas from mice with the indicated administration (left) and quantification of elastin degradation grade (right, n=6). Nonparametric Kruskal-Wallis test with a Dunn multiple comparisons test was applied for comparison of the elastin degradation grade. Scale bar, 50 μm. The black arrows indicate the fragmented elastin. F, Representative images of immunofluorescence staining and quantification for CD68 (biomarker of macrophages)-positive (red) in aortas from mice with the indicated administration (n=10 for BAPN+TSA and n=6 for the other groups). Two-way ANOVA was applied to compare the macrophage infiltration. Nuclei were stained with DAPI (4',6-diamidino-2-phenylindole; blue). Scale bar, 25 μm. The white arrows indicate CD68-positive cells. Animal studies were approved by the Animal Care and Use Committee of Peking University. All data have been analyzed for normality and equal variance as a justification for using parametric analysis. Statistical analysis was performed using GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA). Ad indicates adventitia; Arch, aortic arch; Asc, ascending; Des, descending; and Lu, lumen.
To further examine effects of pan-HDAC inhibitors on TAAD progression, we explored another 2 pan-HDAC inhibitors vorinostat (SAHA) and trichostatin A (TSA) in BAPN-induced TAAD model. Consequently, both TSA and SAHA significantly increased the incidence of BAPN-induced TAAD (BAPN+TSA, 79% [11/14; P=0.0053]; BAPN+SAHA, 67% [10/15; P=0.036]) and subsequently exacerbated aortic elastin degradation and macrophage infiltration as well (Figure [B, C, E, and F]). Inhibition of the HDAC activity has been proposed as a promising therapeutic avenue in the treatment of cancers or cardiovascular diseases. Selective HDAC inhibitors such as class I selective HDAC inhibitors MCT-1 and MS-275 and class II selective HDAC inhibitor MC-1568 have been reported to reduce the pathogenesis and progression of abdominal aortic aneurysm in Ang II–infused ApoE−/− mice.5 However, our results identified that MS-275 and MC-1568 had no effect on BAPN-induced TAAD (Figure [B through D]). Beyond the HDAC family members targeted by MS-275 and MC-1568, pan-HDAC inhibitors additionally inhibit HDAC2, 8, 10, and 11. The inhibition of these HDACs might mediate the pathogenic effect of pan-HDAC inhibitors in TAAD, which requires further investigation.
Our study pointed out that pan-HDAC inhibitors like panobinostat, TSA, and SAHA increased the susceptibility of TAAD in mice. Of note, panobinostat, TSA, and SAHA alone did not induce spontaneous TAAD in mice, but each of them increased the incidence and severity of BAPN-induced TAAD. In accordance, our finding urged that the patients with TAAD or individuals at risk for developing TAAD should be precautious with pan-HDAC inhibitor treatment in their medical therapies.
Article Information
Acknowledgments
Q. Cui and W. Kong designed the experiments. C. Huang curated the data set and performed the bioinformatics analysis with supervision from Q. Cui. S. Zhang and Z. Liu conducted the animal experiments and other chemical experiments with supervision from F. Yu and Y. Fu. Z. Liu helped in elastic Van Gieson staining. S. Zhang, Z. Liu, N. Xie, and W. Kong wrote the manuscript. All authors read and approved the final manuscript.
Sources of Funding
This research was supported by funding from the National Natural Science Foundation of China (81730010, 91839302, 81921001, and 31930056) and the National Key R&D Program of China (2019YFA0801600).
Disclosures
None.
Footnotes
S. Zhang, Z. Liu, and N. Xie contributed equally.
For Sources of Funding and Disclosures, see page 2850.
Contributor Information
Shumin Zhang, Email: 18435147203@163.com.
Zhujiang Liu, Email: liuzhujiang@bjmu.edu.cn.
Nan Xie, Email: xienan@bjmu.edu.cn.
Chuanbo Huang, Email: huangchuanbo@bjmu.edu.cn.
Zhiqing Li, Email: lizhiqing@bjmu.edu.cn.
Fang Yu, Email: vanessa_f@163.com.
Yi Fu, Email: yi.fu@bjmu.edu.cn.
References
- 1.Daneman N, Lu H, Redelmeier DA. Fluoroquinolones and collagen associated severe adverse events: a longitudinal cohort study. BMJ Open. 2015;5:e010077. doi: 10.1136/bmjopen-2015-010077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee CC, Lee MT, Chen YS, Lee SH, Chen YS, Chen SC, Chang SC. Risk of aortic dissection and aortic aneurysm in patients taking oral fluoroquinolone. JAMA Intern Med. 2015;175:1839–1847. doi: 10.1001/jamainternmed.2015.5389 [DOI] [PubMed] [Google Scholar]
- 3.LeMaire SA, Zhang L, Luo W, Ren P, Azares AR, Wang Y, Zhang C, Coselli JS, Shen YH. Effect of ciprofloxacin on susceptibility to aortic dissection and rupture in mice. JAMA Surg. 2018;153:e181804. doi: 10.1001/jamasurg.2018.1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, et al. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929–1935. doi: 10.1126/science.1132939 [DOI] [PubMed] [Google Scholar]
- 5.Galán M, Varona S, Orriols M, Rodríguez JA, Aguiló S, Dilmé J, Camacho M, Martínez-González J, Rodriguez C. Induction of histone deacetylases (HDACs) in human abdominal aortic aneurysm: therapeutic potential of HDAC inhibitors. Dis Model Mech. 2016;9:541–552. doi: 10.1242/dmm.024513 [DOI] [PMC free article] [PubMed] [Google Scholar]