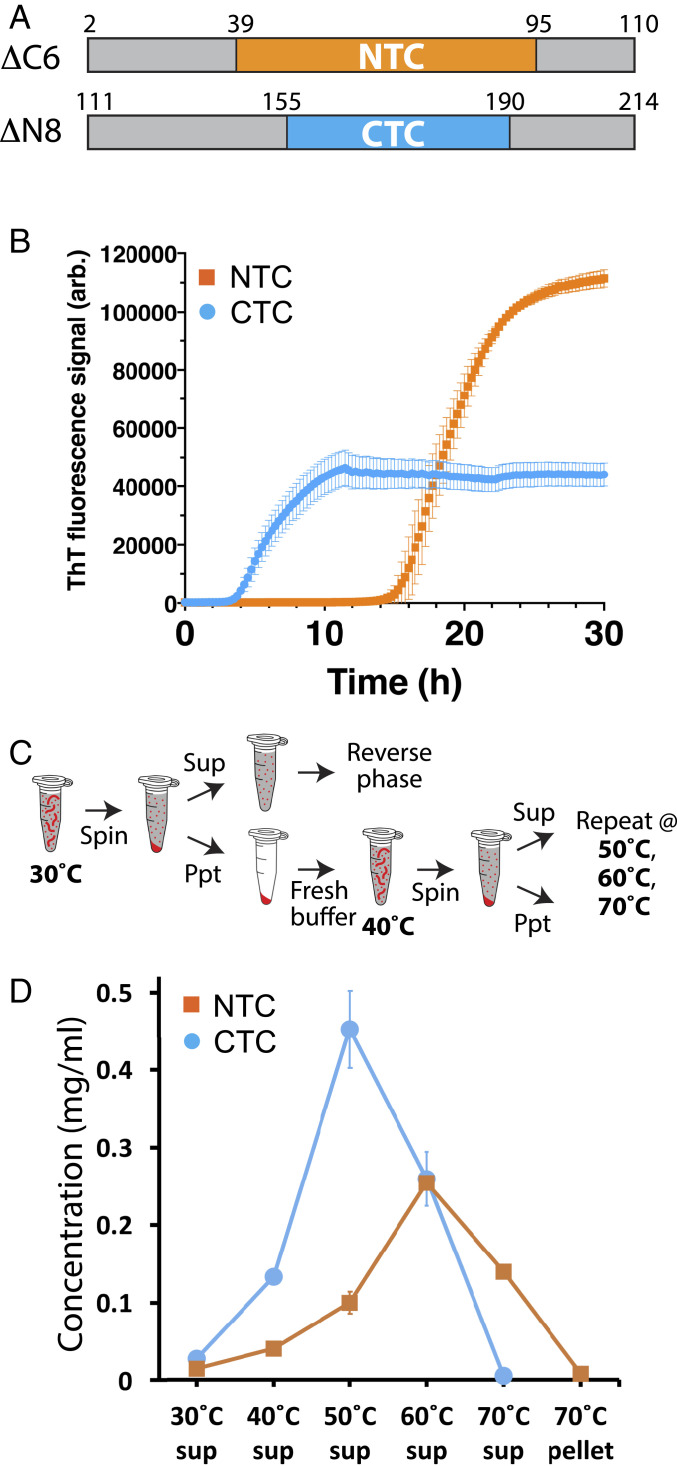
Fig. 5.
Measurements of polymerization rates and stabilities of polymers formed from the N- and C-terminal halves of the FUS low-complexity domain. (A) Schematic diagram of protein fragments corresponding to N- and C-terminal halves of the FUS low-complexity domain. Boxed region shown in yellow corresponds to structural boundaries of the NTC of the FUS LC domain (residues 39 to 95). The boxed region shown in cyan corresponds to functional boundaries of the CTC-forming region of the FUS LC domain (residues 155 to 190). (B) Protein fragments shown in A were expressed in bacteria, purified, and incubated under conditions of neutral pH and physiological monovalent salt for 30 h. Thioflavin-T fluorescence (y axis) was measured as a function of time (x axis). (C) Schematic diagram depicting methods used to monitor the release of monomer subunits from existing polymers as a function of temperature. Existing polymers were incubated at 30 °C and isolated from solution by ultracentrifugation. The supernatant was analyzed by reverse-phase chromatography to quantify soluble monomeric proteins. Pelleted polymers were resuspended in fresh buffer, incubated at 40 °C, and subjected to the same process of centrifugation and supernatant analysis. This process was repeated at 50 °C, 60 °C, and 70 °C. (D) Polymerized samples of NTC and CTC polymers were incubated at varying temperatures (x axis) and monitored for the release of soluble monomers by reverse-phase column chromatography (y axis) as described in C. Little monomeric protein was observed to be released from polymers incubated at 30 °C. CTC polymers released more monomers than NTC polymers at 40 °C and 50 °C and were completely solubilized at 60 °C. NTC polymers required incubation at 70 °C to effect complete solubilization. sup: supernatant.