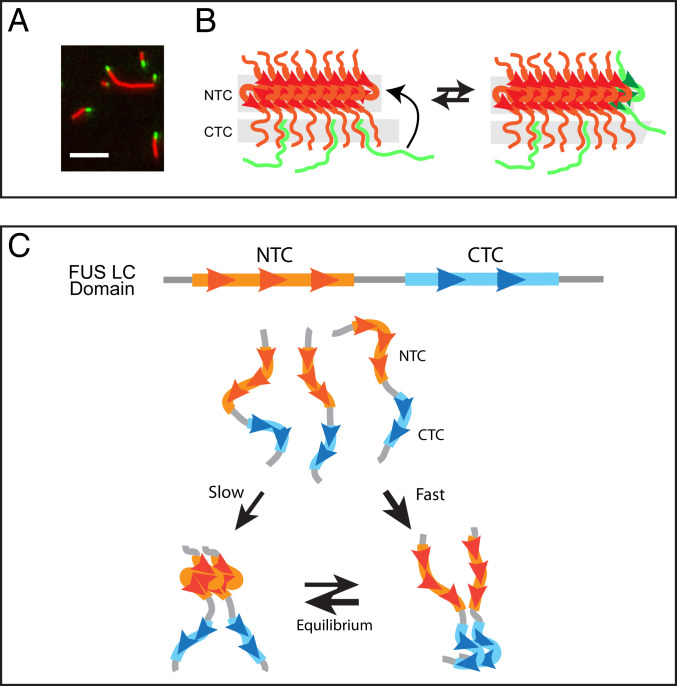
Fig. 8.
Hypothetical pathway of FUS low-complexity domain binding to hydrogel polymers and conceptual pathway for initial self-association of soluble monomers. (A) TIRF microscopic imaging of mCherry:FUS polymers incubated with GFP:FUS monomers. Copolymerization of GFP test protein into existing mCherry:FUS polymers can be seen as green tips extending from red fibrils. (B) Schematic conceptualization of the process of copolymerization. Existing FUS polymer (red) is interpreted to initially bind soluble FUS monomers (green) via interactions wherein the CTC of test proteins attempts to form cross-β interaction with lateral surfaces of existing polymers (Left). Initial, unstable interaction is interpreted to be in equilibrium with copolymerization onto the N-terminal cross-β core (Right). (C) Soluble, unstructured FUS low-complexity domain monomers (Top) are interpreted to preferentially self-associate via C-terminal cross-β core interactions (Right). Slower yet more stable N-terminal cross-β self-association (Left) is proposed to isomerize from dimers held together by the less stable, C-terminal cross-β core. Simultaneous formation of N- and C-terminal cross-β interactions within a single polypeptide is interpreted to be impermissible.