Figure 4.
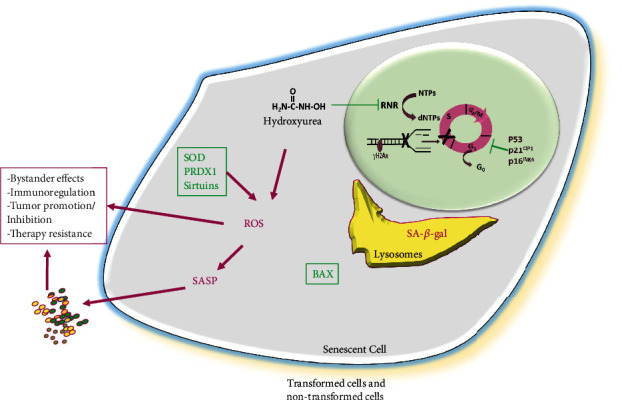
Overview of the main features of hydroxyurea-induced cellular senescence. Hydroxyurea, by inhibition of ribonucleotide reductase (RNR), dramatically reduces the synthesis of deoxyribonucleotides (dNTPs) from ribonucleotide substrates (NTPs). This dNTP pool reduction provokes a termination of DNA replication and may result in replication fork collapse. Furthermore, because of genotoxic HU action, DNA damage is generated, and phosphorylated histone H2AX (γH2AX) binding to DNA breaks is promoted. Cells may suffer an arrest at the S cell cycle phase, concomitant with increased expression of cell cycle inhibitors p16INK4A, p21Cip1, and p53, reinforcing the cell cycle inhibition. During senescence induction, cell size is enlarged, and lysosomal biogenesis is increased, as indicated by elevated levels of expression and senescence-associated-β-galactosidase (SA-β-gal). Along with DNA replication inhibition, augmentation of oxidative stress occurs as reactive oxygen species (ROS) expression levels are elevated, consistently reducing antioxidative stress protein superoxide dismutase (SOD) 2, peroxiredoxin (PRDX) 1, and Sirtuins that contribute to maintaining increased oxidative stress. Moreover, HU-induced senescent cells are refractory to apoptosis, in part from reduced expression of the proapoptotic BAX protein. Senescent cells are metabolically active, and they express and release a set of factors as part of the senescence-associated secretory phenotype (SASP). The SASP may profoundly influence surrounding cells and tissues through increased local and systemic inflammation and regulation of immune response, depending on SASP pattern, positively or negatively affecting tumor growth, and may also contribute to therapy resistance. Magenta words mean increased expression. Magenta arrows mean induction. Gree T-shape symbols mean inhibition. Green words mean reduced expression.
