Figure 1.
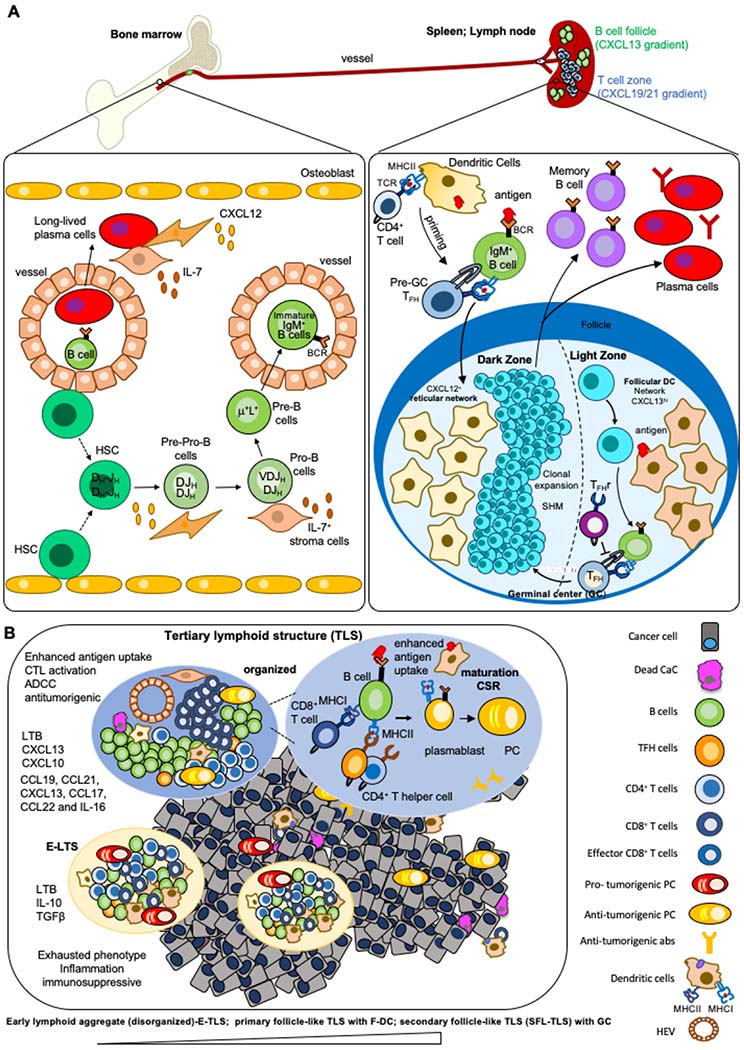
A) The development and differentiation of B cells. B cells develop in the bone marrow. During development, early B-cells similarly rearrange both of their IgH alleles before proceeding to rearrange their IgL alleles. V(D)J recombination in bone marrow pro-B cells first assembles IgH V(D)J exons leading to μ chain expression. Individual B cells express either Igκ or Igλ. Pre-pro-B cells associate with CXCL12hi reticular cells, whereas pro-B cells move towards IL-7-expressing cells like MSC and stroma cells. B cells expressing cell-surface IgM exit the bone marrow and enter the blood to reach the spleen or lymph nodes, where they mature into peripheral mature B cells. In secondary lymphoid tissues, the germinal center is built within the B cell follicles upon infection or immunization. The GC is divided into two distinct compartments. The dark zone (DZ) contains a network of CXCL12-producing reticular cells and is the site of GC B cell proliferation and somatic hypermutation (SHM). B cells follow a CXCL13 gradient to enter the light zone (LZ) through expression of CXCR5. In the LZ, B cells capture antigen presented on follicular dendritic cells (FDCs) which they internalize, process and subsequently present to T follicular helper (TFH) cells in order to undergo selection. This process is regulated by T follicular regulatory (TFr) cells which are also present in the LZ. Cytokines and soluble factors secreted by TFH define isotype CSR. Upon receiving survival signals from TFH cells, B cells re-enter the DZ for further rounds of proliferation and SHM after which they exit the GC as memory B cells or high-affinity antibody-secreting plasma cells. Long-lived plasma cells reside in the bone marrow in direct contact to bone marrow stromal cells. B) The composition and function of tertiary lymphoid structures in cancer. TLS are formed in inflamed sites upon contact between lymphoid tissue organizer IL7+ stromal cell and a lymphoid tissue inducer cell (LTi) in a CXCL13 rich environment. Tissue resident monocytic cells, TH17 or B cells can function as LTi. CCL21 and CXCL12 participate in lymphocytes recruitment, while CXCL13 and CCL19, together with adhesion molecules, control the structural organization of the forming TLS. The infiltrating immune cells form aggregates with a T cell rich zone where mature DC present MHC-Cl II peptides to CD4+T cells, promoting their activation, proliferation and differentiation into T effector cells, including TFH expressing the CXCL13 receptor, CXCR5. The TFH migrate to the CXCL13-rich B cell zone where they induce the activation, proliferation and differentiation of antigen-specific B cells into plasmablasts or their migration into follicles to form GCs where B cells undergo CSR. TLS include a series of heterogenous structures from lymphoid aggregates of T and B cells to structures including follicles of B cells (disorganized to organized). TLS range from the early loose lymphoid aggregates (disorganized, E-TLS) without FDCs to primary follicle-like TLS (PFL-TLS) containing a network of CD21+ FDCs and secondary follicle-like TLS (SFL-TLS) containing a GC including a network of CD21+CD23+ FDCs, as described for secondary lymphoid tissues above. The activation and differentiation of T cells within TLS allows for modulation of their effector function in the TME. In SFL-TLS, CD20+B cells are located within and at the periphery of GCs, similar to GCs in LN. Analysis of the BCR repertoire in follicles, compared with peripheral B cells, reveals clonal expansion, suggesting the development of an antigen-driven B-cell response occurring within TLS-GC. B cells that successfully capture antigen and receive CD40-mediated help from TFH survive and exit the GC as long-lived plasma cells or memory B cells. A fraction of the affinity matured B cells also undergo AID-dependent CSR that regulates isotype switching. Recent work suggests that this process could occur prior to differentiation of B cells into GC B cells or plasmablasts, rather than within GC as previously suggested. The question, if and at which stage of tumor-infiltrating TLSs maturation CSR appears, remains unknown.
